Figure 5.
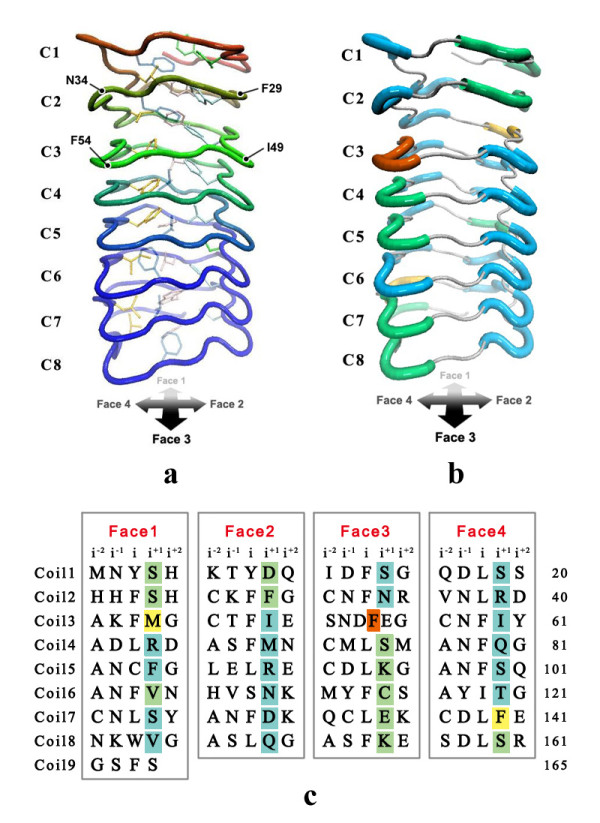
The structure of QnrC protein (residue 1 to 165) based on homology modeling. (a) Tube representation with all side chains of residues at position i. The structure adopts a β-helix fold. Leu and Phe at i positions are well aligned at Face 1, 2, 3 and 4. (b) and (c) illustrate the distribution of type II (in blue) and type IV (in green) β-turns formed by residues at position i+1, i+2 and the residue at position i-2 in the subsequent pentapeptide in the model structure (b) and in sequence (c). The turn involved in the hexapeptide segment (F54 to C57) is in orange. The two turns (M44 to C46 and F140 to N142) that switch between type II and type IV frequently during the structure optimization MD simulation are highlighted in yellow.
