Figure 1. Insulin signaling via PI3K to nuclear and lipoprotein assembly compartments in hepatocytes.
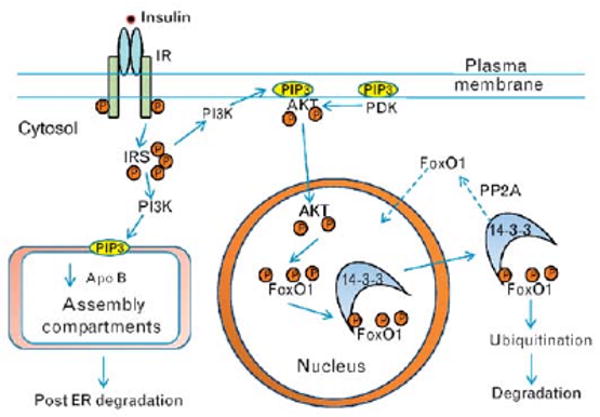
Glucose-stimulated insulin secretion results in high portal insulin that binds to hepatic insulin receptors and activates the tyrosine kinase of the β-subunit leading to autophosphorylation of the receptor and amplification of the tyrosine kinase activity. Activated insulin receptor results in multiple tyrosine phosphorylation of IRS which mediate the activation of PI3K in plasma membranes and in endoplasmic reticulum membranes, both sites containing substrates. PI3K activity results in the production of patches of phosphatidyinositide 3,4,5 tris phosphate (PIP3). In the endoplasmic reticulum, PIP3 interacts with the machinery involved in the assembly of TRLs leading to decreased apoB both by reducing apoB synthesis and by enhancing post-endoplasmic reticulum, presecretory proteolysis, and degradation within lysosomes. In the plasma membrane, PIP3 patches attract the serine/threonine kinase Akt (protein kinase B) through binding to its pleckstrin homology domain bringing Akt in proximity to phosphoinositide-dependent kinase (PDK) which lead to double phosphorylation of Akt (T308, S473) and Akt activation. Activated Akt in turn phosphorylates FoxO1 (Thr-24, Ser-256, Ser-319) with the net result of exclusion of FoxO1 from the nucleus and inactivation of FoxO1 transcriptional activity. Phosphorylated FoxO1 interacts with 14-3-3 protein which helps remove phosphorylated FoxO1 from the nucleus, and leads to FoxO1 ubiquitination and ultimately FoxO1 degradation through the 26S proteasome. IRS, insulin receptor substrates; PDK, phosphoinositide-dependent kinase; PI3K, phosphoinositide 3-kinase; TRL, triglyceride-rich lipoprotein.
