Figure 2. Assembly of hepatic apoB-containing lipoproteins.
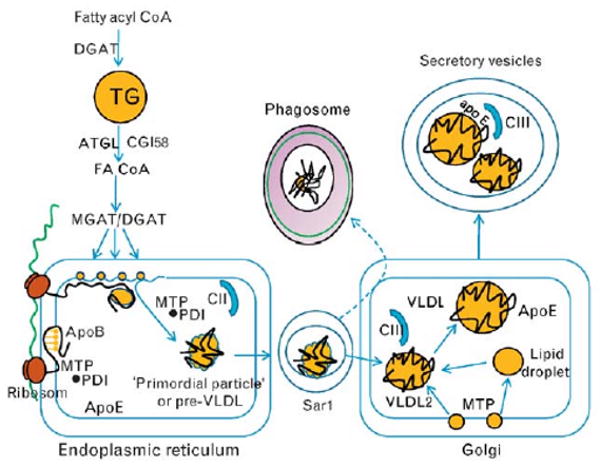
Triglycerides provide lipid precursors for the assembly of apoB-containing lipoproteins. FAs enter the liver and are first converted to fatty acyl CoA derivatives before entering triglyceride droplets for storage through the action of DGAT. Droplets are not assembled into lipoproteins en bloc, but undergo lipolysis and released FAs are re-esterified into triglyceride nucleation sites near the site of lipoprotein assembly in the endoplasmic reticulum. Lipolysis of lipid droplets occurs mainly through ATGL along with a coactivator, abhydrolase domain containing 5 (ABHD5, CGI58, comparative genome identification 58) which releases FAs that are re-esterified by monoacylglyceride and diacylglyceride acyl transferase (MGAT/DGAT) into nucleation sites within the endoplasmic reticulum membrane. The biogenesis of VLDL is initiated by the translation of apoB mRNA on ribosomes docked to the endoplasmic reticulum followed by translocation of the nascent apoB chain through the translocation channel. The first 20% of the N-terminal apoB peptide contains the lipoprotein-initiating domain which interacts with neutral lipid moieties within the inner leaflet. Thereafter, the orderly and sequential appearance of apoB peptide dictates subsequent interactions with MTP and its small MTP subunit, PDI. MTP acts as a chaperone for the N-terminal as lipoprotein formation proceeds, whereas shuttling neutral lipids (triglyceride and cholesteryl esters) and phospholipids from nucleation sites as the core expands into a primordial particle or pre-VLDL. Final phospholipid transfer to the particle by MTP releases through desorption the primordial particle as a secretion-competent nascent lipoprotein. Movement of lipoprotein particles from the endoplasmic reticulum into the secretory pathway involves Sar1-mediated liberation of COPII vesicles. The final maturation of VLDL takes place in the Golgi in which VLDL acquires additional triglyceride possibly through the action of MTP as well as lipoprotein surface components, apoE and apoCIII. ApoCIII blocks lipolytic action of lipoprotein lipase on VLDL triglyceride following VLDL secretion inhibiting the production of cholesteryl ester-rich LDL particles containing only apoB-100 as a core protein. ATGL, adipose triglyceride lipase; FAs, fatty acids; MTP, microsomal triglyceride transfer protein; PDI, protein disulfide isomerase.
