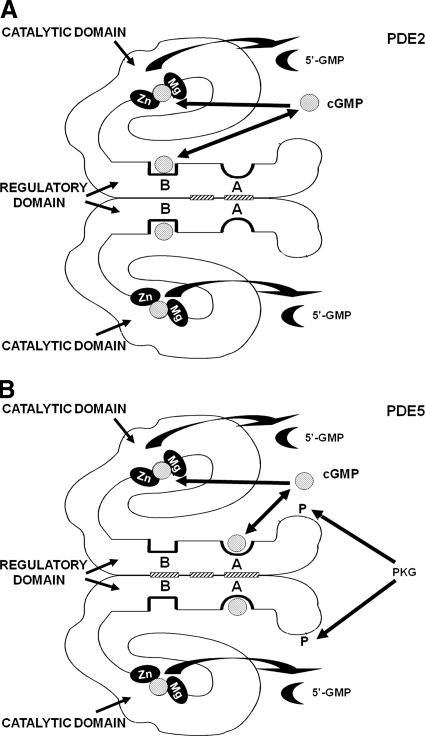
Fig. 7.
Working model of PDE2 and PDE5. A, PDE2 hydrolyzes cGMP and cAMP, and binding of cGMP to GAF-B stimulates cGMP breakdown at the catalytic site, resulting in a negative feedback action of cGMP level. Zn2+ and another divalent metal ion (perhaps Mg2+ or Mn2+) comprise a binuclear metal binding site where a hydroxyl ion from water is polarized for breaking the cyclic phosphate ring. B, PDE5 has overall structure similar to that of PDE2. PKGI phosphorylates Ser-102 located near the amino terminus; both phosphorylation and allosteric cGMP binding, which occurs at GAF-A, increase cGMP breakdown at subsaturating cGMP level. [Adapted from Francis SH and Corbin JD (2005) Phosphodiesterase-5 inhibition: the molecular biology of erectile function and dysfunction. Urol Clin North Am 32:419–429. Copyright © 2005 Elsevier Ltd. Used with permission.]