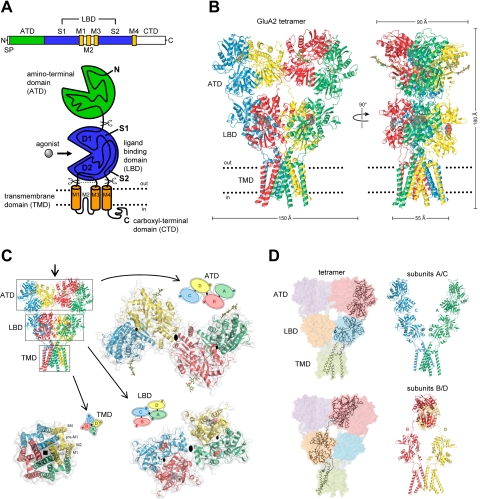
Fig. 1.
Structure and domain organization of glutamate receptors. A, linear representation of the subunit polypeptide chain and schematic illustration of the subunit topology. Glutamate receptor subunits have a modular structure composed of two large extracellular domains [the ATD (green) and the LBD (blue)]; a TMD (orange) that forms part of the ion channel pore; and an intracellular CTD. The LBD is defined by two segments of amino acids termed S1 and S2. The TMD contains three membrane-spanning helices (M1, M3, and M4) and a membrane re-entrant loop (M2). The isolated S1 and S2 segments have been constructed by deleting the ATD along with the TMD and joining S1 and S2 with a hydrophilic linker (dotted line). SP, signal peptide. B, crystal structure at 3.6 Å of the membrane-spanning tetrameric GluA2 AMPA receptor (PDB code 3KG2). C, subunit interfaces between the ATD, LBD, and TMD of the four subunits in the membrane-spanning tetrameric GluA2 AMPA receptor. The subunits are viewed from top down the 2-fold axis of symmetry. The ATDs and LBDs have a 2-fold axis of symmetry, whereas the TMDs have 4-fold axis of symmetry. D, the symmetry mismatch between the TMDs and the extracellular domains (ATDs and LBDs) as well as the subunit crossover (or domain swapping) from the LBD to the ATD give rise to two distinct types of subunits in the homotetrameric GluA2 receptor with two distinct conformations. The subunits are referred to as the A/C and B/D subunits. [Adapted from Sobolevsky AI, Rosconi MP, and Gouaux E (2009) X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462:745–756. Copyright © 2009 Nature Publishing Group. Used with permission.]