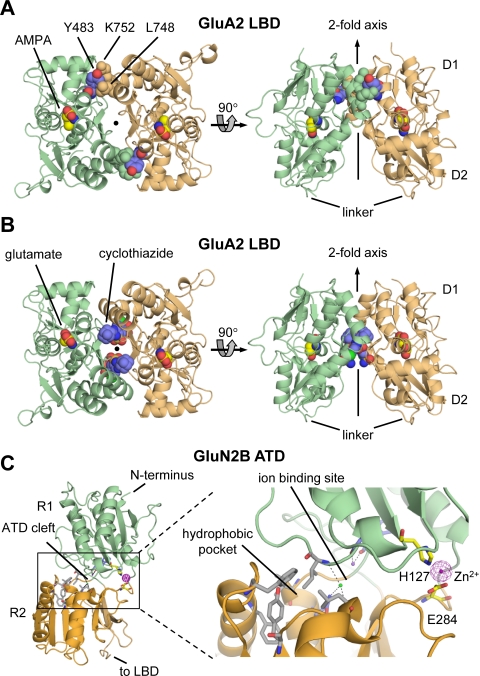
Fig. 10.
Allosteric regulation of glutamate receptors. A, the structure of the dimer formed between LBDs of the L483Y mutated GluA2 (PDB code 1LB8) is shown from the top (left) and perpendicular (right) to the 2-fold axis. Mutation of residue 483 (blue) located on D1 from Leu to Tyr attenuates desensitization and stabilizes the dimer interface by interactions with Leu748 and Lys752 on the opposing protomer. B, the LBD dimer interface contains two binding sites for the positive AMPA receptor modulator cyclothiazide (blue) that inhibits receptor desensitization (PDB code 1LBC). Cyclothiazide stabilizes the dimer interface by forming additional intersubunit interactions in the dimer interface. C, structure of the GluN2B ATD with bound Zn2+ (PDB code 3JPY). The cleft formed by the upper R1 and the lower R2 lobes can be divided into three pockets: the hydrophobic pocket (gray carbon atoms), the ion binding site with Na+ and Cl−, and the hydrophilic pocket with the Zn2+ binding site. The hydrophobic pocket is thought to bind ifenprodil and its analogs.