Fig. 1.
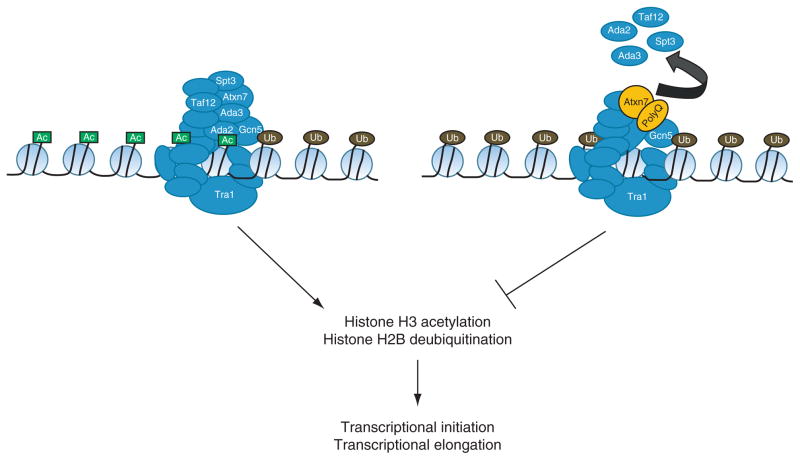
Incorporation of polyglutamine-expanded ATXN7 into SAGA complexes alters subunit composition and catalytic activities. SAGA complexes containing polyglutamine-expanded ATXN7 exhibit reduced levels of Ada2, Ada3, Spt3, and TAF12. Several of these subunits, such as Ada2 and Ada3, are necessary for the ability of Gcn5 to acetylate nucleosomal histones. While experiments have not yet addressed the issue, there is potential for polyglutamine expansion of ATXN7 to disrupt the histone deubiquitination activity of SAGA complexes since the N-terminal region of ATXN7 is involved in the attachment of the deubiquitination module to the greater complex. Both histone acetylation and deubiquitination are involved in the initiation of gene transcription and subsequent elongation by RNA polymerase II.