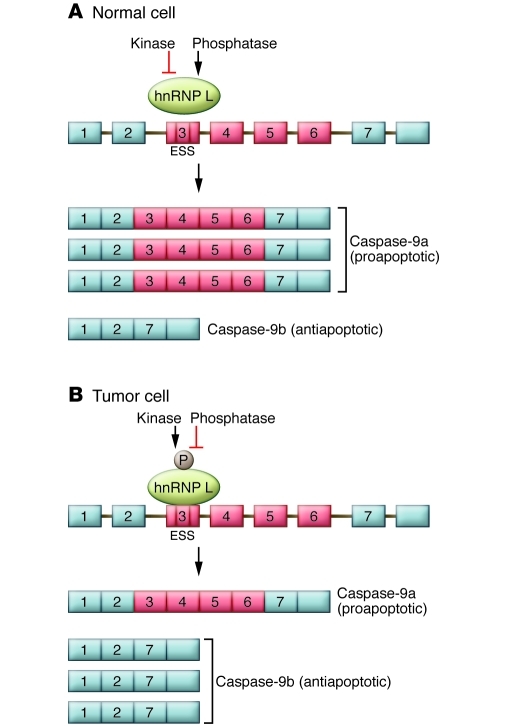
Figure 2. Model for the regulation of caspase-9 mRNA expression by hnRNP L.
The caspase-9 pre-mRNA contains four variable exons (exons 3, 4, 5, and 6) that are differentially included or excluded to generate the longer caspase-9a or shorter caspase-9b mRNAs. (A) The work of Goehe et al. (7) indicates that in normal cells, a higher ratio of caspase-9a/9b mRNA is present, which leads to increased levels of the proapoptotic caspase-9a protein isoform. (B) In tumor cells, the four variable exons are skipped, leading to a lower ratio of caspase-9a/9b mRNA, which leads to increased levels of the antiapoptotic caspase-9b protein isoform. Goehe et al. show that regulation of the alternative RNA processing pattern of the caspase-9 mRNA is achieved by the differential phosphorylation of the hnRNP L protein. hnRNP L protein is largely unphosphorylated in normal cells, while in NSCLC cells, hnRNP L is phosphorylated at specific serine, threonine, and tyrosine residues. The differential phosphorylation pattern of hnRNP L could be regulated by cell type–specific kinases and/or phosphatases. Phosphorylation of hnRNP L Ser52, specifically in tumor cells, regulates the interaction of hnRNP L with caspase-9 RNA and regulates differential RNA processing of this transcript, as discussed in the text. ESS, exonic splicing silencer.