Fig. 5. EKLF recruits the activity of NFκB modulation in activated but not resting macrophages.
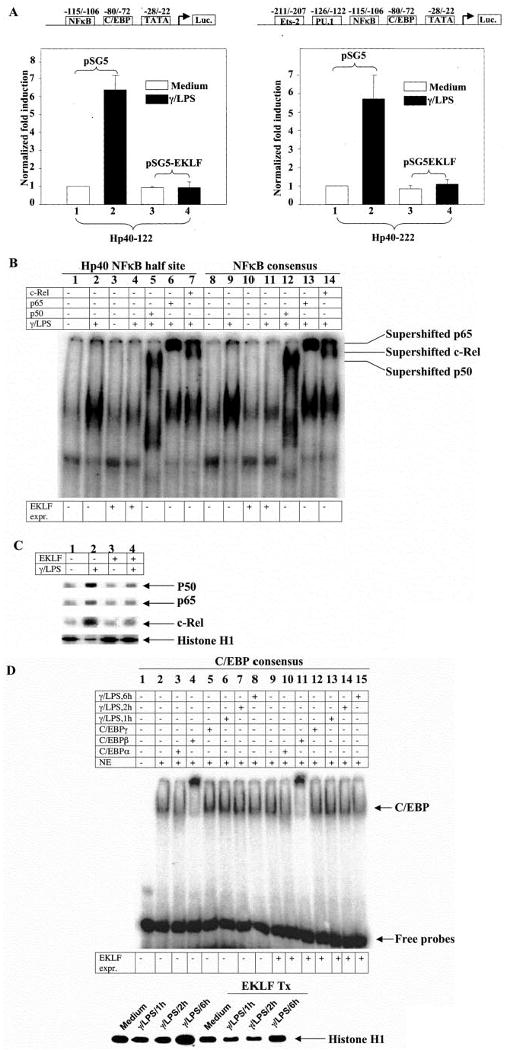
A, the effect of EKLF overexpression is shown on the luciferase reporter construct driven by Hp40–122 and Hp40–222 that harbor a putative NFκB half-site binding site TGAAATTCCCC (−116 to −106). Hp40–122 and Hp40–222 were derived from wild type Hp40 promoter with the sequences downstream of nucleotides −122 to −222 (relative to transcription start site), respectively. Transfection efficiency was normalized against co-transfected CMV-β-galactosidase plasmids. The mean value of the basal unstimulated promoter activity was set at 1. The transcriptional activity was expressed as fold induction from the unstimulated promoter activity. n = 4. B, supershift EMSA using the Hp40 NFκB half-site oligonucleotides and the NFκB consensus oligonucleotides is shown with the nuclear extracts from EKLF-enriched RAW264.7 cells. Cells were stimulated by murine IFN-γ (10 ng/ml) overnight followed by LPS (1 μg/ml) for 1 h. The experiment shown is representative of three similar experiments. C, the same nuclear extracts were immunoblotted with anti-p50, stripped, and re-probed with anti-p65 and anti-c-Rel, respectively. Re-probing with anti-histone H1 was performed as a control. The experiment shown is representative of three similar experiments. D, supershift EMSA using the C/EBP consensus oligonucleotides is shown with the nuclear extracts from EKLF-enriched RAW264.7 cells. Cells were stimulated by murine IFN-γ (10 ng/ml) overnight followed by LPS (1μg/ml) for 1, 2, or 6 h. Nuclear extracts were immunoblotted with anti-histone H1 as a control. The experiment shown is representative of three similar experiments.
