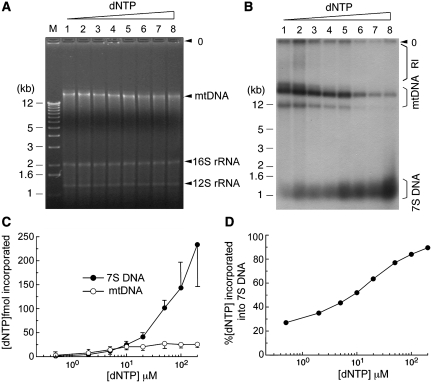
Figure 3.
Influence of nucleotide concentration on in vitro synthesis of 7S DNA and mtDNA. Replication reactions were performed with HeLa CE (∼1.5 × 105 cell equivalent) in the presence of variable equimolar dNTP levels: lane 1, 0.5 µM dNTP/5 µCi α-32P-dATP; lanes 2–4, 2, 5 or 10 µM dNTP/10 µCi α-32P-dATP; lanes 5–7, 20, 50 or 100 µM dNTP/20 µCi α-32P-dATP; lane 8, 200 µM dNTP/40 µCi α-32P-dATP. For each reaction, the specific radioactivity per µM dNTP is estimated as follows: lane 1, 2.2 × 107 cpm; lane 2, 1.1 × 107 c.p.m.; lane 3, 4.4 × 106 c.p.m.; lanes 4–5, 2.2 × 106 c.p.m.; lane 6, 8.8 × 105 c.p.m.; lanes 7–8, 4.4 × 105 c.p.m. After 2 h incubation, reaction mixtures were resolved on a 1% agarose gel, as described in ‘Materials and Methods' section. Position of mtDNA and two mitochondrial ribosomal DNAs, 16S and 12S rRNA, are indicated in (A). Immediately after photography, the gel was dried and then subjected to autoradiography to detect newly synthesized 7S DNA, mtDNA and replication intermediates (RI), as shown in (B). Nucleotide incorporation into mtDNA and 7S DNA was quantified as described in 'Materials and methods' section and average values obtained from four independent experiments are presented in (C). (D) Nucleotide incorporation into 7S DNA is presented relative to total dNTP incorporation (i.e. 7S DNA + full-length mtDNA).