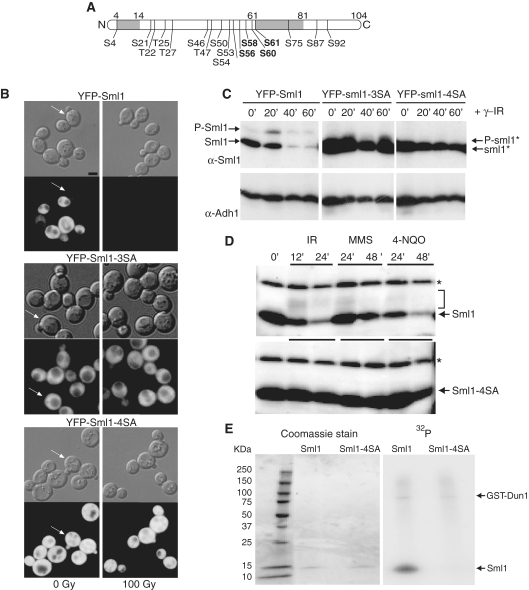
Figure 1.
Mutations that eliminate putative phosphorylation sites in Sml1 stabilize the protein after DNA damage treatment and prevent in vivo and in vitro phosphorylation. (A) Depiction of the Sml1 protein with the positions of all serine and threonine residues that are potential phosphorylation sites. The shaded regions indicate the positions of two α helices, including the C-terminal helix, which is important for Sml1 binding to Rnr1. The four serines changed in the sml1-4SA mutant are shown in bold type. (B) Mid-log phase cultures of cells expressing YFP-Sml1 (W4622-14B), YFP-sml1-3SA (W6976-4A) or YFP-sml1-4SA (W4748-4D) fusion proteins were treated with 100 Gy of γ-irradiation. Protein stability was examined by visualizing YFP fluorescence before and after treatment. White arrows indicate cells that are in S phase (small buds). The scale bar is equal to 3 µm. (C) Total yeast extracts of the strains shown in (B) were probed with anti-Sml1 antibody to examine stability and in vivo phosphorylation, as determined by mobility shift of immunoblot, of the fusion proteins in logarithmically growing cultures. To control for loading, the membrane was stripped and re-probed using anti-Adh1 antibody. (D) Total yeast extracts from wild-type (W1588-4C) and sml1-4SA (W3329-7D) strains were examined for Sml1 in vivo phosphorylation in response to treatment with γ-irradiation (300 Gy), 0.05% MMS and 4-NQO (0.25 mg/l). The arrow indicates the position of Sml1 proteins. The slower migrating bands (indicated by a bracket) are due to phosphorylation (21). Immunoblots were probed with anti-Sml1 serum. The top band, labeled with an asterisk, is a Sml1-independent cross-reacting band used as a loading control. (E) Recombinant purified Sml1 and sml1-4SA were incubated with GST-Dun1 fusion protein purified from yeast. A portion of the reaction was resolved on a 4–20% SDS–PAGE gradient gel, stained with Coomassie blue and subsequently visualized by autoradiography for 32P incorporation. Both reactions contained the same amount of recombinant protein (left) and exhibited the same level of kinase activity as observed by GST-Dun1 autophosphorylation (right).