Abstract
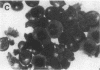
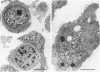
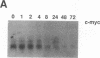
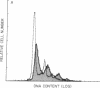
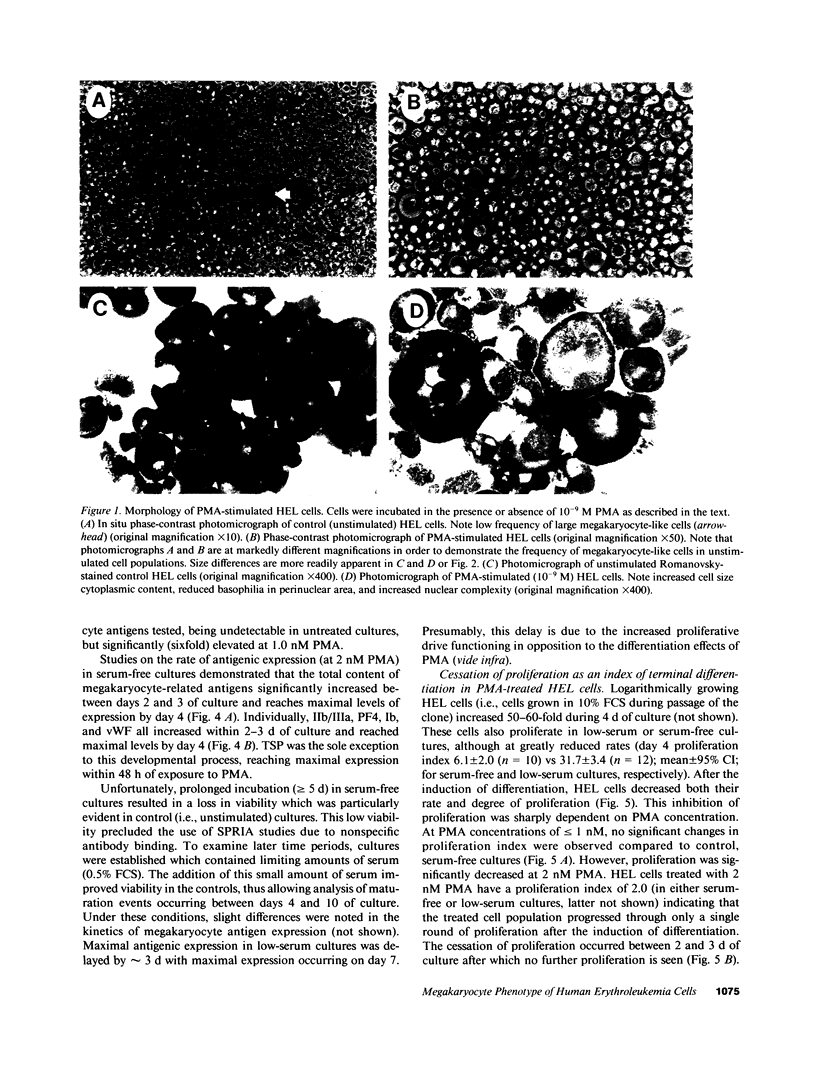
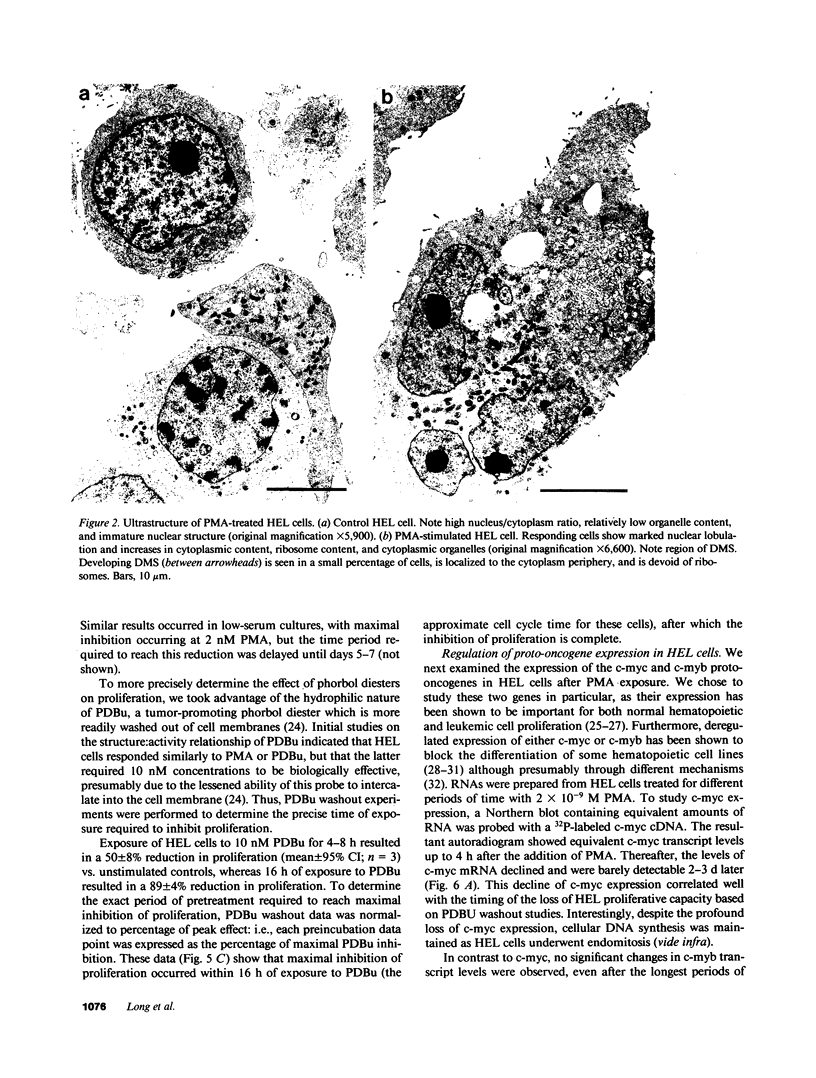
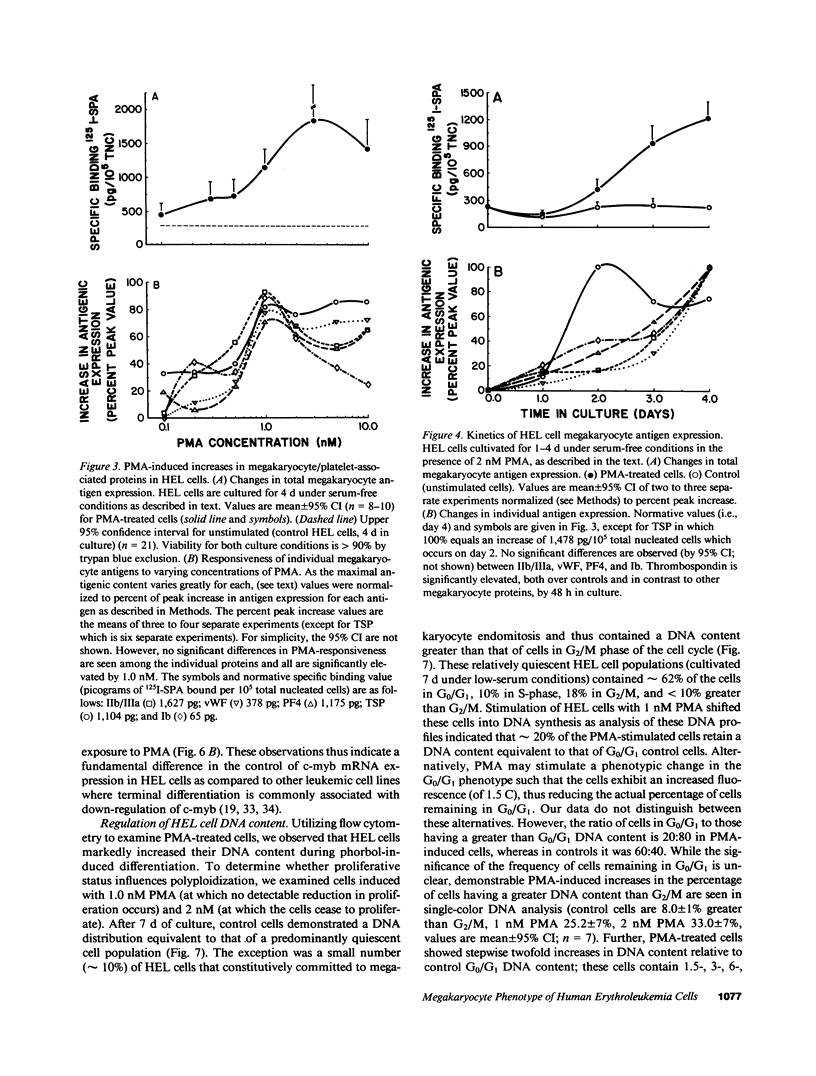
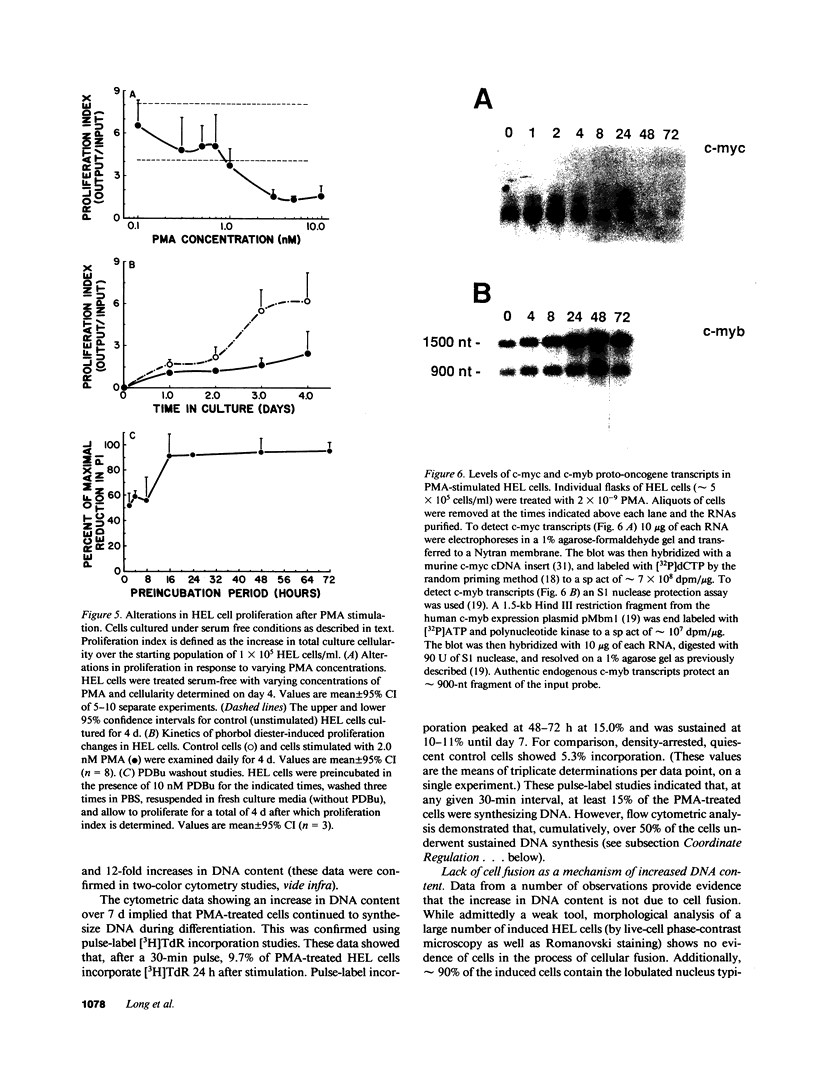
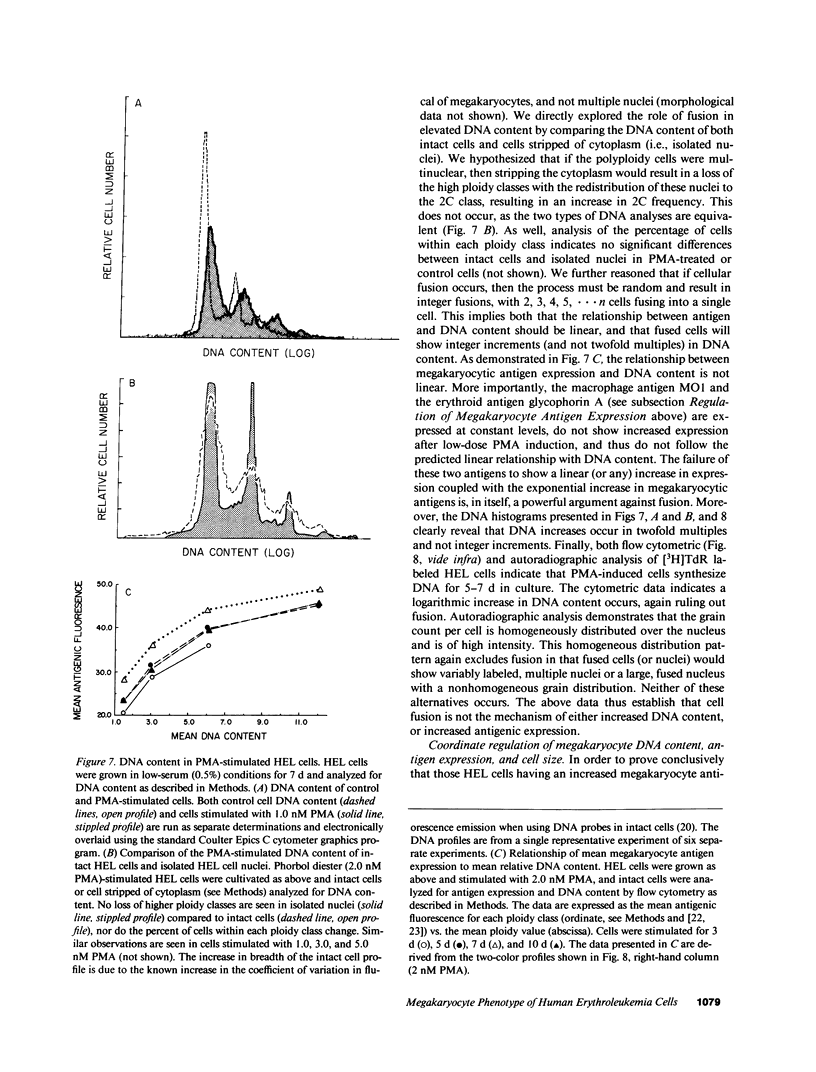
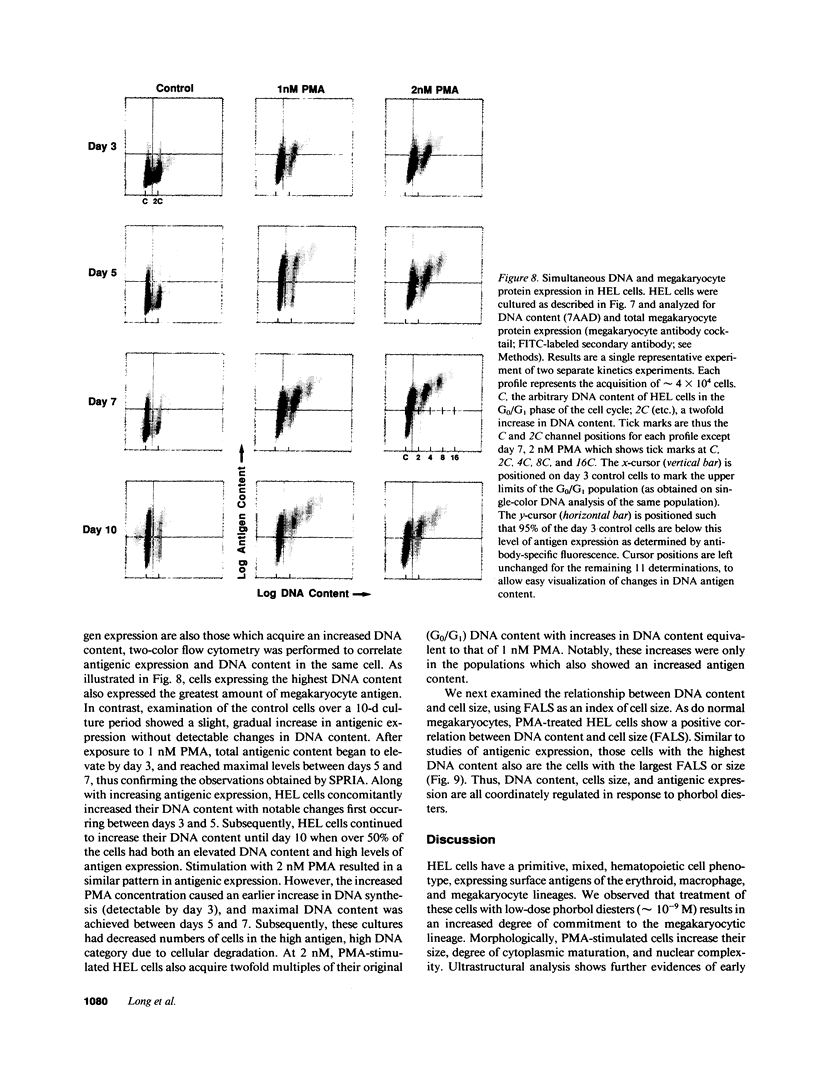
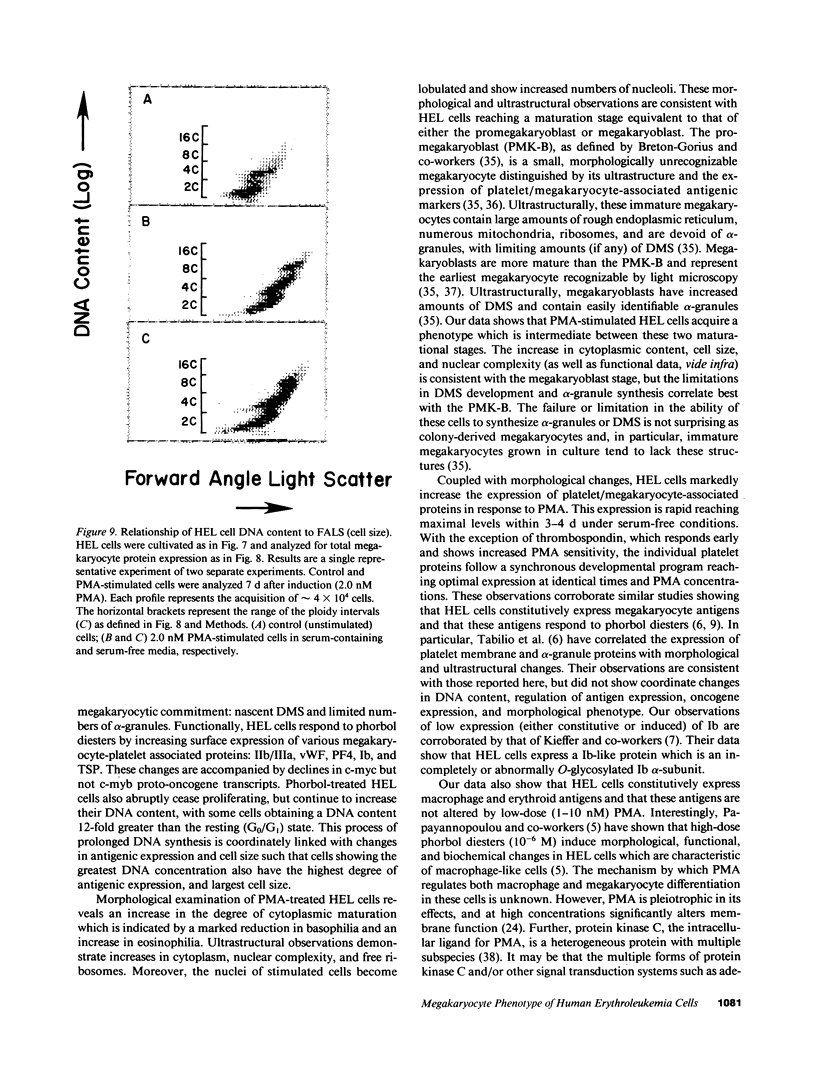
Induction of human erythroleukemia (HEL) cells with nanomolar tumor-promoting phorbol myristate acetate (PMA) diesters results in the synchronous acquisition of multiple markers of the megakaryocyte phenotype. Induced cells markedly increase their content of cytoplasm and show features of morphological maturation. At the ultrastructural level, PMA-treated cells show increases in cytoplasm, nuclear lobulation and nucleolar content, and free ribosomes. Limited numbers of cells also express alpha-granules and nascent demarcation membrane systems. Functionally, PMA-stimulated HEL cells express increased amounts of the megakaryocyte/platelet proteins: glycoprotein IIb/IIIa, platelet factor 4, von Willebrand factor, glycoprotein Ib, and thrombospondin. No changes are observed in antigenic markers of the erythroid (glycophorin A) or macrophage lineages (MO-1 or MO-2). The increases in antigenic expression are rapid, reaching maximum levels within 3-4 d under serum-free conditions. Treatment with PMA also abruptly (within 1-2 d) inhibits cellular division in these cells. Washout studies indicate that phorbols exert their effect within 18-24 h, the approximate cell cycle time for these cells. Consistent with proliferative arrest, c-myc proto-oncogene transcripts begin to decline within 8 h of PMA treatment, although transcripts of c-myb are unaffected. Importantly, megakaryocyte differentiation is associated with endomitotic DNA synthesis (i.e., continued DNA synthesis in the absence of mitosis and cytokinesis), with HEL cells reaching a DNA content of 3-12 times that of unstimulated cells. Endomitosis is coordinately regulated with changes in antigenic expression and cell size such that those cells having the highest DNA content are the largest and also express the greatest levels of antigen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop W. R., Bell R. M. Attenuation of sn-1,2-diacylglycerol second messengers. Metabolism of exogenous diacylglycerols by human platelets. J Biol Chem. 1986 Sep 25;261(27):12513–12519. [PubMed] [Google Scholar]
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Breton-Gorius J., Vainchenker W. Expression of platelet proteins during the in vitro and in vivo differentiation of megakaryocytes and morphological aspects of their maturation. Semin Hematol. 1986 Jan;23(1):43–67. [PubMed] [Google Scholar]
- Bruno E., Miller M. E., Hoffman R. Interacting cytokines regulate in vitro human megakaryocytopoiesis. Blood. 1989 Feb 15;73(3):671–677. [PubMed] [Google Scholar]
- Clarke M. F., Kukowska-Latallo J. F., Westin E., Smith M., Prochownik E. V. Constitutive expression of a c-myb cDNA blocks Friend murine erythroleukemia cell differentiation. Mol Cell Biol. 1988 Feb;8(2):884–892. doi: 10.1128/mcb.8.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classon M., Henriksson M., Sümegi J., Klein G., Hammarskjöld M. L., Hammaskjöld M. L. Elevated c-myc expression facilitates the replication of SV40 DNA in human lymphoma cells. Nature. 1987 Nov 19;330(6145):272–274. doi: 10.1038/330272a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987 Nov;70(5):1233–1244. [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Corash L., Levin J., Mok Y., Baker G., McDowell J. Measurement of megakaryocyte frequency and ploidy distribution in unfractionated murine bone marrow. Exp Hematol. 1989 Mar;17(3):278–286. [PubMed] [Google Scholar]
- Dmitrovsky E., Kuehl W. M., Hollis G. F., Kirsch I. R., Bender T. P., Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986 Aug 21;322(6081):748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- Ebbe S. Biology of megakaryocytes. Prog Hemost Thromb. 1976;3:211–229. [PubMed] [Google Scholar]
- FEINENDEGEN L. E., ODARTCHENKO N., COTTIER H., BOND V. P. Kinetics of megacaryocyte proliferation. Proc Soc Exp Biol Med. 1962 Oct;111:177–182. doi: 10.3181/00379727-111-27738. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gewirtz A. M., Calabretta B. A c-myb antisense oligodeoxynucleotide inhibits normal human hematopoiesis in vitro. Science. 1988 Dec 2;242(4883):1303–1306. doi: 10.1126/science.2461588. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Greenberg S. M., Rosenthal D. S., Greeley T. A., Tantravahi R., Handin R. I. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988 Dec;72(6):1968–1977. [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Itani T., Yamaguchi M., Ariga H. c-myc protein can be substituted for SV40 T antigen in SV40 DNA replication. Nucleic Acids Res. 1987 Jun 25;15(12):4889–4899. doi: 10.1093/nar/15.12.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer N., Debili N., Wicki A., Titeux M., Henri A., Mishal Z., Breton-Gorius J., Vainchenker W., Clemetson K. J. Expression of platelet glycoprotein Ib alpha in HEL cells. J Biol Chem. 1986 Dec 5;261(34):15854–15862. [PubMed] [Google Scholar]
- Kreutter D., Caldwell A. B., Morin M. J. Dissociation of protein kinase C activation from phorbol ester-induced maturation of HL-60 leukemia cells. J Biol Chem. 1985 May 25;260(10):5979–5984. [PubMed] [Google Scholar]
- Lachman H. M., Cheng G. H., Skoultchi A. I. Transfection of mouse erythroleukemia cells with myc sequences changes the rate of induced commitment to differentiate. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6480–6484. doi: 10.1073/pnas.83.17.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Levine R. F., Fedorko M. E. Isolation of intact megakaryocytes from guinea pig femoral marrow. Successful harvest made possible with inhibitions of platelet aggregation; enrichment achieved with a two-step separation technique. J Cell Biol. 1976 Apr;69(1):159–172. doi: 10.1083/jcb.69.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W., Gragowski L. L., Heffner C. H., Boxer L. A. Phorbol diesters stimulate the development of an early murine progenitor cell. The burst-forming unit-megakaryocyte. J Clin Invest. 1985 Aug;76(2):431–438. doi: 10.1172/JCI111990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W., Heffner C. H. Detection of human megakaryocyte antigens by solid-phase radioimmunoassay. Exp Hematol. 1988 Jan;16(1):62–70. [PubMed] [Google Scholar]
- Long M. W., Heffner C. H., Gragowski L. L. Cholera toxin and phorbol diesters synergistically modulate murine hematopoietic progenitor cell proliferation. Exp Hematol. 1988 Mar;16(3):195–200. [PubMed] [Google Scholar]
- Long M. W., Hutchinson R. J., Gragowski L. L., Heffner C. H., Emerson S. G. Synergistic regulation of human megakaryocyte development. J Clin Invest. 1988 Nov;82(5):1779–1786. doi: 10.1172/JCI113791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W. Signal transduction events in in vitro megakaryocytopoiesis. Blood Cells. 1989;15(1):205–229. [PubMed] [Google Scholar]
- Long M. W., Smolen J. E., Szczepanski P., Boxer L. A. Role of phorbol diesters in in vitro murine megakaryocyte colony formation. J Clin Invest. 1984 Nov;74(5):1686–1692. doi: 10.1172/JCI111585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W., Williams N., Ebbe S. Immature megakaryocytes in the mouse: physical characteristics, cell cycle status, and in vitro responsiveness to thrombopoietic stimulatory factor. Blood. 1982 Mar;59(3):569–575. [PubMed] [Google Scholar]
- Long M. W., Williams N. Immature Megakaryocytes in the Mouse: Morphology and quantitation by acetylcholinesterase staining. Blood. 1981 Nov;58(5):1032–1039. [PubMed] [Google Scholar]
- Lu L., Briddell R. A., Graham C. D., Brandt J. E., Bruno E., Hoffman R. Effect of recombinant and purified human haematopoietic growth factors on in vitro colony formation by enriched populations of human megakaryocyte progenitor cells. Br J Haematol. 1988 Oct;70(2):149–156. doi: 10.1111/j.1365-2141.1988.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Marie J. P., Vernant J. P., Dreyfus B., Breton-Gorius J. Ultrastructural localization of peroxidases in 'undifferentiated' blasts during the blast crisis of chronic granulocytic leukaemia. Br J Haematol. 1979 Dec;43(4):549–558. doi: 10.1111/j.1365-2141.1979.tb03787.x. [DOI] [PubMed] [Google Scholar]
- Martin P., Papayannopoulou T. HEL cells: a new human erythroleukemia cell line with spontaneous and induced globin expression. Science. 1982 Jun 11;216(4551):1233–1235. doi: 10.1126/science.6177045. [DOI] [PubMed] [Google Scholar]
- Nakaki T., Mita S., Yamamoto S., Nakadate T., Kato R. Inhibition by palmitoylcarnitine of adhesion and morphological changes in HL-60 cells induced by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1984 May;44(5):1908–1912. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Odell T. T., Jr, Jackson C. W., Gosslee D. G. Maturation of rat megakaryocytes studied by microspectrophotometric measurement of DNA. Proc Soc Exp Biol Med. 1965 Aug-Sep;119(4):1194–1199. doi: 10.3181/00379727-119-30412. [DOI] [PubMed] [Google Scholar]
- Odell T. T., Jr, Jackson C. W. Polyploidy and maturation of rat megakaryocytes. Blood. 1968 Jul;32(1):102–110. [PubMed] [Google Scholar]
- Ogura M., Morishima Y., Ohno R., Kato Y., Hirabayashi N., Nagura H., Saito H. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood. 1985 Dec;66(6):1384–1392. [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Kurachi S., Nelson R. Analysis of the erythroid phenotype of HEL cells: clonal variation and the effect of inducers. Blood. 1987 Dec;70(6):1764–1772. [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Kurachi S., Tweeddale M., Messner H. Surface antigenic profile and globin phenotype of two new human erythroleukemia lines: characterization and interpretations. Blood. 1988 Sep;72(3):1029–1038. [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Yokochi T., Chait A., Kannagi R. Human erythroleukemia cell line (HEL) undergoes a drastic macrophage-like shift with TPA. Blood. 1983 Oct;62(4):832–845. [PubMed] [Google Scholar]
- Papayannopoulou T., Raines E., Collins S., Nakamoto B., Tweeddale M., Ross R. Constitutive and inducible secretion of platelet-derived growth factor analogs by human leukemic cell lines coexpressing erythroid and megakaryocytic markers. J Clin Invest. 1987 Mar;79(3):859–866. doi: 10.1172/JCI112895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Yokochi T., Nakamoto B., Martin P. The surface antigen profile of HEL cells. Prog Clin Biol Res. 1983;134:277–292. [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J., Rodgers C. c-myc antisense transcripts accelerate differentiation and inhibit G1 progression in murine erythroleukemia cells. Mol Cell Biol. 1988 Sep;8(9):3683–3695. doi: 10.1128/mcb.8.9.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochownik E. V. c-myb but not c-myc suppresses the hemin-induced nonterminal expression of hemoglobin by murine erythroleukemia cells. Blood. 1989 Feb 15;73(3):782–786. [PubMed] [Google Scholar]
- Rabinovitch P. S., Torres R. M., Engel D. Simultaneous cell cycle analysis and two-color surface immunofluorescence using 7-amino-actinomycin D and single laser excitation: applications to study of cell activation and the cell cycle of murine Ly-1 B cells. J Immunol. 1986 Apr 15;136(8):2769–2775. [PubMed] [Google Scholar]
- Sledge G. W., Jr, Glant M., Jansen J., Heerema N. A., Roth B. J., Goheen M., Hoffman R. Establishment in long term culture of megakaryocytic leukemia cells (EST-IU) from the marrow of a patient with leukemia and a mediastinal germ cell neoplasm. Cancer Res. 1986 Apr;46(4 Pt 2):2155–2159. [PubMed] [Google Scholar]
- Stanton L. W., Fahrlander P. D., Tesser P. M., Marcu K. B. Nucleotide sequence comparison of normal and translocated murine c-myc genes. Nature. 1984 Aug 2;310(5976):423–425. doi: 10.1038/310423a0. [DOI] [PubMed] [Google Scholar]
- Tabilio A., Rosa J. P., Testa U., Kieffer N., Nurden A. T., Del Canizo M. C., Breton-Gorius J., Vainchenker W. Expression of platelet membrane glycoproteins and alpha-granule proteins by a human erythroleukemia cell line (HEL). EMBO J. 1984 Feb;3(2):453–459. doi: 10.1002/j.1460-2075.1984.tb01827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Alvarez P. A., Brott D. A., Liu D. Y. Bacterial lipopolysaccharide, phorbol myristate acetate, and muramyl dipeptide stimulate the expression of a human monocyte surface antigen, Mo3e. J Immunol. 1985 Dec;135(6):3869–3877. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Schlossman S. F. Analysis of antigenic determinants on human monocytes and macrophages. Blood. 1982 Apr;59(4):775–786. [PubMed] [Google Scholar]
- Tomer A., Harker L. A., Burstein S. A. Flow cytometric analysis of normal human megakaryocytes. Blood. 1988 May;71(5):1244–1252. [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]
- de Leval M. Etude cytochimique quantitative des acides désoxyribonucléiques au cours de la maturation mégacaryocytaire. Nouv Rev Fr Hematol. 1968 May-Jun;8(3):392–394. [PubMed] [Google Scholar]