Abstract
It was recently reported that levo-tetrahydropalmatine (l-THP), a dopamine (DA) D1 and D2 receptor antagonist purified from the Chinese herb Stephanie, appears to be effective in attenuating cocaine self-administration, cocaine-triggered reinstatement and cocaine-induced conditioned place preference in preclinical animal models. The present study was designed to contrast l-THP's effects on cocaine self-administration under fixed-ratio (FR) and progressive-ratio (PR) reinforcement, and to study l-THP's effects on cocaine-enhanced brain stimulation reward (BSR). Systemic administration of l-THP produced dose-dependent, biphasic effects, i.e., low-to-moderate doses (1, 3, 10 mg/kg) increased, while a high dose (20 mg/kg) inhibited cocaine self-administration behavior under FR2 reinforcement. The increased cocaine self-administration is likely a compensatory response to a reduction in cocaine's rewarding effects, because the same low doses of l-THP dose-dependently attenuated cocaine self-administration under PR reinforcement and also attenuated cocaine-enhanced BSR. These attenuations of PR cocaine self-administration and cocaine-enhanced BSR are unlikely due to l-THP-induced sedation or locomotor inhibition, because only 10 mg/kg, but not 1-3 mg/kg of l-THP inhibited locomotion, sucrose self-administration and asymptotic operant performance in the BSR paradigm. In vivo microdialysis demonstrated that l-THP slightly elevates extracellular nucleus accumbens DA by itself, but dose-dependently potentiates cocaine-augmented DA, suggesting that a postsynaptic, rather than presynaptic, DA receptor antagonism underlies l-THP's actions on cocaine reward. Together, the present data, combined with previous findings, support the potential use of l-THP for treatment of cocaine addiction.
Keywords: cocaine, levo-tetrahydropalmatine, dopamine, self-administration, brain reward, addiction
1. Introduction
Cocaine addiction is a persistent social and health problem worldwide, with no effective medications currently available for treatment. In light of the important role of the mesolimbic dopamine (DA) system in drug abuse (Wise, 2005), much attention has focused on various DA receptor antagonists as potential treatments (Rothman and Glowa, 1995; Platt et al., 2002). However, clinical trials with D1 or D2 receptor antagonists for cocaine addiction have failed due to either ineffectiveness, surmountability by increased drug intake, and/or unfavorable side-effects such as dysphoria, extrapyramidal movements or inhibition of natural reward (Sherer et al., 1989; Nann-Vernotica et al., 2001; Haney et al., 2001; Newton et al., 2001; see reviews by Platt et al., 2002; Gorelick et al., 2004).
Alternatively, a number of research groups have investigated novel compounds that target other DA receptors, such as D3 receptors (Heidbreder et al., 2005; Xi and Gardner, 2007) or drug cocktails that target multiple DA receptors. The rationale for the development of D3 receptor antagonists is largely based on the highly restricted distribution of D3 receptors in brain reward circuits (Sokoloff et al., 2006), suggesting that D3 receptor antagonists may have anti-cocaine actions but without significant side-effects. The drug cocktail medications are based on the presumption that multiple DA receptor blockade may produce additive or synergistic therapeutic effects, but have less unwanted side-effects due to lower doses of each component in the cocktails (Soyka and De Vry, 2000; Platt et al., 2002; Gorelick et al., 2004).
Levo-tetrahydropalmatine (l-THP) is a purified active ingredient from the Chinese herb Stephanie (Jin, 1987; Jin et al., 2002). l-THP, as a traditional sedative-analgesic agent, has been used for more than 40 years in China for the treatment of chronic pain and anxious insomnia (Jin, 1987; Jin et al., 2002). Recent studies have demonstrated that l-THP is a non-selective D1 and D2 (and possibly D3) receptor antagonist (Jin et al, 1986; Xu et al., 1989; Guo et al., 1997; Mantsch et al., 2007). In addition, it also has high binding affinity for α1- and α2-adrenergic receptors and several serotonin (5-hydroxytryptamine; 5-HT) receptors (5-HT1A, 5-HT4 and 5-HT7) (Mantsch et al., 2007; Shijiang Li at the Medical College of Wisconsin, unpublished data). Given that cocaine is also a non-selective monoamine (DA, norepinephrine and 5-HT) reuptake inhibitor (Wise, 2005), the similar binding properties of l-THP for monoamine receptors suggest that l-THP may act as a natural cocktail-like cocaine antagonist. Recent studies have shown that l-THP significantly inhibits cocaine- or methamphetamine-induced conditioned place preference (Ren et al., 2000; Luo et al., 2003), cocaine self-administration under fixed-ratio (FR) reinforcement and cocaine-triggered reinstatement of drug-seeking behavior in rats (Mantsch et al., 2007). In addition, l-THP also inhibits opiate tolerance and withdrawal syndromes in rats (Jin et al., 1998; Ge et al., 1999), as well as locomotor sensitization to oxycodone, an opioid receptor agonist, in mice (Liu et al., 2005). Further, it was recently reported that l-THP significantly attenuates opiate craving and relapse in heroin addicts (Yang et al., 2006). However, it remains unclear whether l-THP inhibits the acute rewarding effects of cocaine, or whether such an inhibition is due to a direct action on brain reward function or to non-specific sedation and locomotor inhibition. Therefore, in the present study, we investigated: 1) whether l-THP inhibits cocaine self-administration under both FR and progressive-ratio (PR) reinforcement, and cocaine-enhanced brain stimulation reward (BSR). Both PR self-administration and BSR are thought to be sensitive and reliable paradigms to evaluate a drug's rewarding effects (Gardner, 2000; Wise, 2005); 2) whether the same doses of l-THP inhibit locomotion or sucrose self-administration; 3) whether l-THP itself has rewarding or aversive effects in the BSR paradigm; and finally, 4) whether a presynaptic or postsynaptic DA receptor mechanism underlies l-THP's potential therapeutic actions by measuring brain DA responses to cocaine in the presence or absence of l-THP using in vivo brain microdialysis.
2. Methods
2.1. Animals
Experimentally naïve male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA) weighing 250 to 300 g were used for all experiments. They were housed individually in a climate-controlled animal colony room on a reversed light-dark cycle (lights on at 7:00 PM, lights off at 7:00 AM) with free access to food and water. The animals were maintained in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the U.S. National Academy of Sciences, and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse of the U.S. National Institutes of Health.
2.2. Cocaine Self-Administration
Surgery
All animals were prepared for experimentation by surgical catheterization of the right external jugular vein. The venous catheters were constructed of microrenathane (Braintree Scientific Inc., Braintree, MA, USA), and catheterization was performed under sodium pentobarbital anaesthesia (65 mg/kg, i.p.) with standard aseptic surgical techniques. After exiting the jugular, the catheter passed subcutaneously to the top of the skull, where it exited into a connector (a modified 24 gauge cannula; Plastics One, Roanoke, VA, USA) mounted to the skull with jeweler's screws and dental acrylic. During experimental sessions, the catheter was connected to the injection pump via tubing encased in a protective metal spring from the head-mounted connector to the top of the experimental chamber.
Apparatus
Intravenous (i.v.) self-administration experiments were conducted in operant response test chambers (32 × 25 × 33 cm) from MED Associates Inc. (Georgia, VT, USA). Each test chamber had 2 levers: 1 active and 1 inactive, located 6.5 cm above the floor. Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no consequence. A cue light and a speaker were located 12 cm above the active lever. The house light was turned on at the start of each 3 hr test session. To aid acquisition and maintenance of drug self-administration behavior, each drug infusion was always paired with a conditioned cue-light and a cue-sound (tone). Scheduling of experimental events and data collection were accomplished using MED Associates software.
General procedure
After recovery from surgery, each rat was placed into a test chamber and allowed to lever-press for i.v. cocaine (1 mg/kg/infusion) delivered in 0.08 ml over 4.6 sec, on an FR1 reinforcement schedule. During the 4.6 sec infusion time, additional responses on the active lever were recorded but did not lead to additional infusions. Each session lasted 3 hours. FR1 reinforcement was used for 3-5 days until stable cocaine self-administration was established. Then, subjects were randomly assigned to 1 of the following 2 experiments: 1) cocaine self-administration under FR2 reinforcement conditions, or 2) cocaine self-administration under progressive-ratio (PR) reinforcement conditions. In all experiments, l-THP was given 30 min prior to testing because our previous data showed that onset of behavioral effects occurs approximately 30 min after systemic administration of this compound.
Cocaine self-administration under FR2 reinforcement
After transition from FR1 reinforcement, subjects were allowed to continue cocaine (0.5 mg/kg/infusion) self-administration under FR2 reinforcement until the following criteria for stable cocaine-maintained responding were met: less than 10% variability in the inter-response interval and less than 10% variability in number of presses on the active lever for at least 3 consecutive days. The two different sequential doses of cocaine used during i.v. cocaine self-administration acquisition and stabilization were chosen based on previous findings that 1 mg/kg/infusion of cocaine produces rapid and facile acquisition of cocaine self-administration behavior, while 0.5 mg/kg/infusion of cocaine leads to a significant increase in the work demand (i.e., lever presses) for the same amount of cocaine intake, and therefore increases sensitivity of measurements of changes in drug-taking or drug-seeking behavior. To avoid cocaine overdose, each animal was limited to a maximum of 50 cocaine injections per 3hr session. After stable rates of responding were established, each subject randomly received 1 of 4 doses of l-THP (1, 3, 10, 20 mg/kg, i.p.) or vehicle (1 ml sterile water) 30 min prior to the test session. Animals then received an additional 5-7 days of self-administration of cocaine alone until baseline response rate was reestablished prior to testing the next dose of drug. The order of testing for the various doses of l-THP was counterbalanced according to a Latin square design.
Cocaine self-administration under progressive ratio reinforcement
Initial cocaine self-administration under FR1 and FR2 reinforcement was identical to that outlined above. After stable cocaine self-administration under FR2 reinforcement was established, the subjects were switched to cocaine self-administration (0.5 mg/kg/infusion) under a PR schedule, during which the work requirement of lever presses needed to receive a single i.v. cocaine infusion was progressively raised within each test session (see details in Arnold and Roberts, 1997) according to the following PR series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492 and 603 until the breakpoint was reached. The break-point was defined as the maximal work load (i.e., number of lever presses) completed for the last cocaine infusion prior to a 1-hr period during which no infusions were obtained by the animal. Animals were allowed to continue daily sessions of cocaine self-administration under PR reinforcement conditions until day-today variability in break-point fell within 1-2 ratio increments for 3 consecutive days. Once a stable break-point was established, subjects were assigned to 4 subgroups to determine the effects of 3 different doses of l-THP (1, 3, 10 mg/kg, i.p.) or vehicle (1 ml sterile water) on PR break-point for cocaine self-administration. Since it is relatively difficult to re-achieve basal break-point levels after each drug test, a between-subject, rather than a within-subject, design was used to determine the dose-response effects of l-THP on PR break-point.
2.3. Oral sucrose self-administration
The procedures for oral sucrose self-administration were identical to the procedures for cocaine self-administration except for the following: 1) no surgery was performed on the animals in the sucrose self-administration experiment, and 2) active lever presses led to delivery of 0.1 ml of 5% sucrose solution into a liquid food tray on the operant chamber wall. After stable sucrose self-administration under FR2 reinforcement was established, 1 of 3 doses of l-THP (1, 3, 10 mg/kg, i.p.) or vehicle (1 ml sterile water) was administered 30 min prior to sucrose self-administration tests.
2.4. Electrical brain stimulation reward
Surgery
Under the same anesthesia as used above, rats were placed in a stereotaxic frame, and a unilateral monopolar stainless-steel stimulating electrode (Plastics One, Roanoke, VA, USA) was placed into the medial forebrain bundle at the anterior-posterior level of the lateral hypothalamus, using standard aseptic surgical and stereotaxic techniques. The implant coordinates for the tips of the electrodes were AP -2.56, ML ± 1.9, and DV -8.6, according to the rat brain stereotaxic atlas of Paxinos and Watson. The electrode was attached to the skull with jeweler's screws and dental acrylic. A wire leading from the electrode was wrapped around a skull screw to serve as a current return.
Apparatus
The experiments were conducted in standard MED Associates operant chambers (32 × 25 × 33 cm). Each operant chamber had a lever located 6.5 cm above the floor, connected to an electrical stimulator.
General procedure
After 7 days of recovery from surgery, rats were allowed to self-train (autoshape) to lever-press for rewarding BSR. Each press on the lever resulted in a 500-msec train of 0.1-msec rectangular cathodal pulses through the electrode in the rat's medial forebrain bundle, followed by a 500 msec “timeout” in which further presses did not produce brain stimulation. The initial stimulation parameters were 72 Hz and 200 μA. If the animal did not learn to lever-press, the stimulation intensity was increased daily by 50 μA until the animal learned to press (45-60 responses/30 sec) or a maximum of 800 μA was reached. Animals that did not lever-press at 800 μA or in which the stimulation produced unwanted effects (e.g., head or body movements or vocalization) were removed from the experiment.
Rate-frequency BSR procedure
Following establishment of lever-pressing for BSR, animals were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz in descending order. At each pulse frequency, animals responded for 2 30-sec time periods (“bins”), after which the pulse frequency was decreased by 0.05 log units. The response rate for each frequency was defined as the mean number of lever responses during the 2 30-sec bins. Following each 30-sec bin, the lever retracted for 5 sec. Throughout the experiment, animals were run for 3 sessions a day. Since lever-pressing behavior was variable during the first session (the “warm up” session), but was stable during the second and third sessions, the data from the first session were discarded, and the data from the second and third sessions were designated as the baseline session data and test session data, respectively. The BSR threshold (θ0) was defined as the minimum frequency at which the animal responded for rewarding stimulation. BSR threshold (θ0) was mathematically derived for each “baseline” run and each “drug” run by analyzing each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies using “best-fit” mathematical algorithms. Specifically, each rate-frequency BSR function was mathematically fitted, by iterative computer programs derived from the Gauss-Newton algorithm for nonlinear regression, to 3 different sigmoid curve-fitting mathematical growth models that appear to accurately fit rate-frequency brain-stimulation reward functions (Coulombe and Miliaressis, 1987) - the Gompertz model (Y′ = ae-e(b-cX)), the logistic model (Y′ = a/[1+e(b-cX)]), and the Weibull function (Y′ = a[1-e-(bX)c]); where Y′ is the rate of response (number of lever presses for rewarding brain stimulation per unit of time), X is the pulse frequency, and a, b, and c are parameters approximated from each empirical rate-frequency data curve (a representing the asymptotic response rate value, b relating to the intercept of the rate-frequency curve with the Y axis, and c representing the rate at which Y increments). From each curve-fitting model, a solution for θ0 was obtained. Thus, for each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies, 3 solutions for θ0 were obtained. The 3 solutions for θ0 were averaged, to produce a mean θ0 for each rate-frequency BSR function generated by a given animal over a given descending series of pulse frequencies. The mean θ0 values were expressed as means ± S.E.M. Data analyses were performed on percent changes from baseline levels.
Testing the effects of cocaine and/or l-THP on BSR
Once a baseline θ0 value was achieved (<15% variation over 5 continuous days), the effects of cocaine and/or l-THP on BSR were assessed. On test days, animals randomly received 1 of 3 different doses of l-THP (1, 3, 10 mg/kg i.p.), or vehicle (1 ml sterile water) 30 min prior to a cocaine injection (2 mg/kg i.p.). After each test, animals received an additional 5-7 days of BSR re-stabilization until a new baseline θ0 was established. The order of testing for various doses of l-THP was counterbalanced according to a Latin square design. The effect of l-THP on cocaine-enhanced BSR was evaluated by comparing cocaine-induced alterations in θ0 value in the presence or absence of each dose of l-THP pretreatment.
2.5. In vivo Microdialysis
Microdialysis experiments were performed in 4 additional groups of rats to evaluate the effects of l-THP (1, 3, 10 mg/kg) or vehicle on basal levels of extracellular DA and cocaine-enhanced NAc DA. Microdialysis protocols and probe construction were as reported previously (Xi et al., 2006). Guide cannulae (20G, Plastics One, Roanoke, VA) were surgically implanted into the NAc (AP+1.6 mm, ML±1.8 mm, DV-4.3 mm, angled 6° from vertical) using standard surgical and stereotaxic techniques. Microdialysis probes were inserted into the NAc 12 hr before the experiment to minimize damage-induced neurotransmitter release. During the experiment, microdialysis buffer was perfused through the probe (2.0 μl/min) for at least 2 hr before sampling started. Samples were collected every 20 min into 10 μl 0.5 M perchloric acid to prevent neurotransmitter degradation. After 1 hr baseline collection, 1 of 3 doses of l-THP (1, 3, 10 mg/kg i.p.) or vehicle (1 ml sterile water) were administered 40 min prior to cocaine priming. All samples were frozen at -80°C until analyzed.
Microdialysate DA was measured by high performance liquid chromatography (HPLC) with an ESA (ESA Biosciences, Chelmsford, MA) electrochemical (EC) detection system as described previously (Xi et al., 2006), upgraded by a Coulochem III EC detector. Areas under the curve (AUC) for DA were measured and quantified with external standard curves. The minimum detection limit for DA was 1-10 fmol.
2.6. Locomotor activity
Three groups of rats were used to observe the effects of l-THP on spontaneous locomotor activity. On the test day, rats were initially placed in locomotor detection monitors (Accuscan, Columbus, OH) for a 1 hr habituation period, and then each rat was removed and administered one of two doses of l-THP (3, 10 mg/kg i.p.) or vehicle (1 ml sterile water). After drug injection, animals were placed back into the locomotor monitors for 2 hrs to record possible alterations in locomotion after l-THP administration. Total distance was used to evaluate the effects of l-THP on locomotion.
2.7. Drugs
Cocaine HCl (Sigma Chemical Co., Saint Louis, MO, USA) was dissolved in physiological saline. l-THP was obtained from the Beijing Basic Medicine Research Institute (Beijing, China). Sterile water was used as vehicle.
2.8. Data analyses
All data are presented as means (± S.E.M.). One-way analysis of variance (ANOVA) was used to analyze the overall effects of l-THP on cocaine self-administration and cocaine-enhanced BSR or NAc DA (Figs. 1-6, bar figures). Two-way ANOVA with repeated measurements was used to analyze the time courses (Figs. 1B, 5A, 6A, 6C) of the effects of l-THP on cocaine self-administration under FR2 reinforcement and on cocaine-enhanced NAc DA. Post-ANOVA pre-planning individual group comparisons were carried out using the Bonferroni t-test procedure.
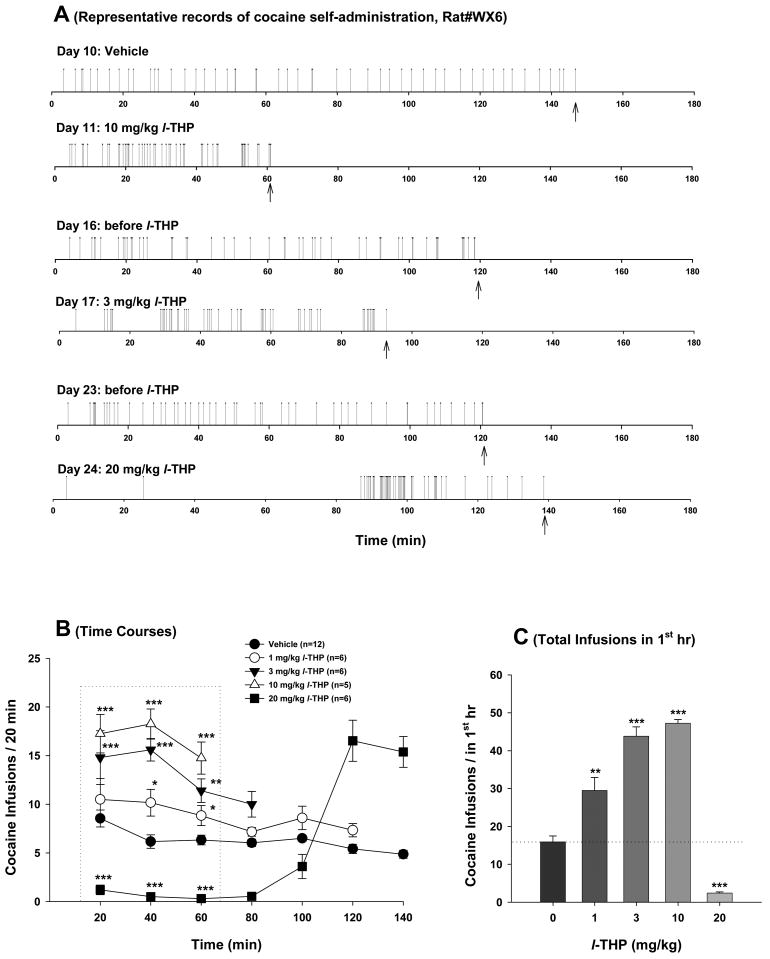
Figure 1.
Effects of l-THP on cocaine self-administration under FR2 reinforcement. Panel A shows representative cocaine self-administration records illustrating that systemic administration of 3 or 10 mg/kg of l-THP increased, while 20 mg/kg inhibited cocaine self-administration. Each vertical line represents a cocaine infusion (0.5 mg/kg/infusion). Panel B shows the mean time courses of cocaine self-administration for the maximal 50 infusions after each dose of l-THP administration. Panel C shows the total numbers of cocaine infusions during the 1st hr of cocaine self-administration. The arrows (↑) indicate the last cocaine infusion. *p<0.05, **p<0.01, ***p<0.001, when compared with the same time point in the vehicle control group (Panel B) or the vehicle (0 mg/kg l-THP) control group (Panel C).
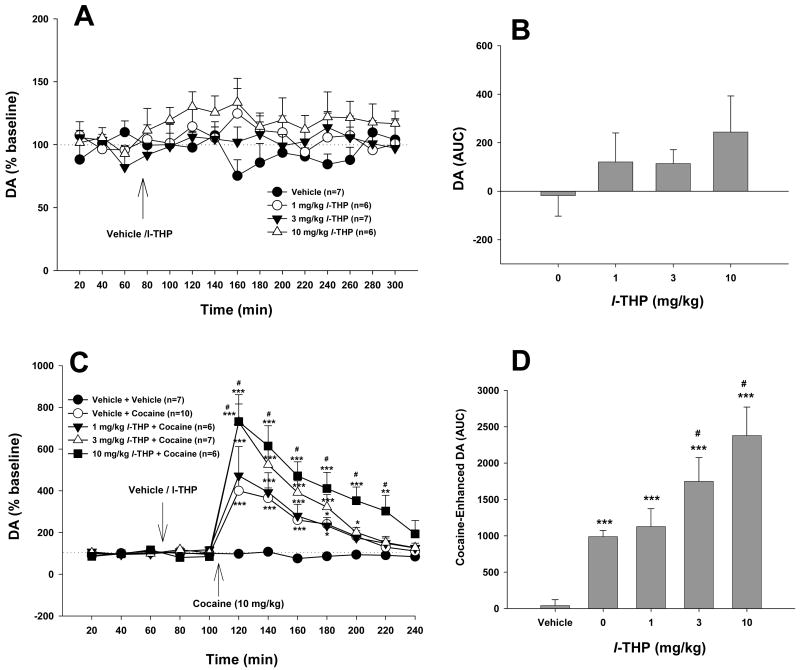
Figure 6.
Effects of cocaine and/or l-THP on extracellular DA in the NAc. L-THP (1, 3, 10 mg/kg) slightly elevated extracellular NAc DA. Pretreatment with l-THP dose-dependently augmented cocaine-enhanced DA in the NAc. *p<0.05, **p<0.01, ***p<0.001, when compared with baselines before l-THP administration (C) or the vehicle (for cocaine) treatment group (D). #p<0.05, when compared with the vehicle (0 mg/kg l-THP) plus cocaine treatment group (C, D).
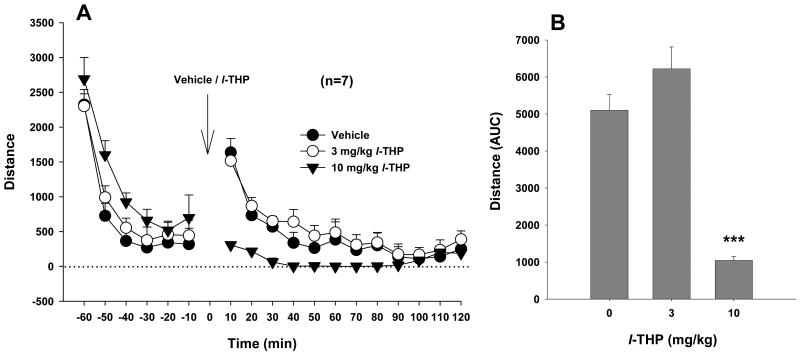
Figure 5.
Effects of l-THP on locomotor activity in rats. L-THP, at 10 mg/kg, but not 3 mg/kg, significantly inhibited basal levels of locomotion. ***p<0.001, when compared with the vehicle control group.
3. Results
3.1. Effects of l-THP on fixed-ratio (FR) cocaine self-administration
To determine whether l-THP altered the rewarding effects of cocaine, we first observed the dose-effects of l-THP on cocaine self-administration under FR2 reinforcement. Figure 1A shows representative cocaine self-administration patterns before and after l-THP administration, illustrating that 3 or 10 mg/kg l-THP significantly increased cocaine self-administration rates, while 20 mg/kg l-THP produced an initial virtually total inhibition (for about 90 min) and a subsequent burst-like increase in cocaine self-administration behavior. Figure 1B shows the averaged time courses of the changes in cocaine self-administration rate (every 20 min), indicating that l-THP produced dose-dependent biphasic effects, i.e., the lower doses (1, 3, 10 mg/kg) increased cocaine self-administration, while the highest dose (20 mg/kg) produced an initial decrease and a subsequent increase in cocaine self-administration behavior. Since we established a maximum of 50 infusions during each 3-hr self-administration session to prevent cocaine overdose, the lower doses (1, 3, 10 mg/kg) of l-THP produced dose-dependent reduction in the durations (time) to complete the maximal 50 cocaine infusions. Two-way ANOVA for repeated measurements for the initial 1 hr self-administration data (Figure 1B) indicated a statistically significant l-THP treatment main effect (F4,16=60.95, p<0.001), but no significant time main effect (F2,8=2.38, p>0.05) or treatment × time interaction (F8,32=1.35, p>0.05). Individual group comparisons using Bonferroni t statistic indicated that l-THP-induced alterations in cocaine self-administration were statistically significant after 1 mg/kg (t=2.43, p<0.05), 3 mg/kg (t=6.52, p<0.001), 10 mg/kg (t=7.54, p<0.001) or 20 mg/kg (t=6.16, p<0.001), when compared with the vehicle control group (Figure 1B). Figure 1C shows the total numbers of cocaine infusions within the first hour of self-administration. One-way ANOVA for repeated measurements indicated a significant l-THP treatment main effect (F4,19=86.55, p<0.001). Post-ANOVA individual group comparisons revealed a statistically significant increase in cocaine self-administration after 1 mg/kg (t=4.71, p<0.01), 3 mg/kg (t=9.67, p<0.001), or 10 mg/kg (t=10.23, p<0.01), and a decrease in cocaine self-administration after 20 mg/kg (t=5.35, p<0.001) l-THP administration.
3.2. Effects of l-THP on progressive-ratio (PR) cocaine self-administration
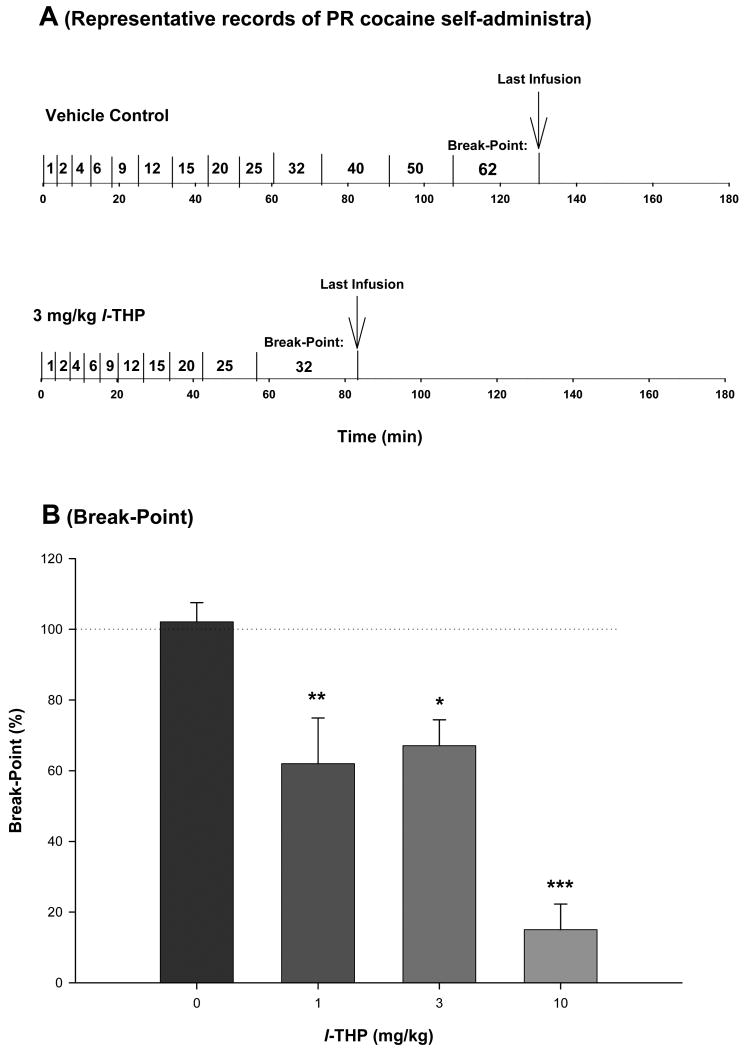
One explanation for the increases of cocaine self-administration under FR2 reinforcement is that lower doses of l-THP produce a partial reduction in cocaine's rewarding effects, which leads to a compensatory increase in cocaine-taking behavior. To test this hypothesis, we studied the effects of l-THP on cocaine self-administration under PR reinforcement conditions. We found that the same doses of l-THP (1, 3, 10 mg/kg) significantly and dose-dependently lowered the break-point for PR cocaine self-administration (Figure 2), suggesting a dose-dependent reduction in cocaine's rewarding efficacy after l-THP administration. Figure 2A shows representative records of cocaine self-administration under PR reinforcement, indicating that 3 mg/kg l-THP lowered the break-point from 62 after vehicle to 32 after l-THP administration. One-way ANOVA for the mean break-point data shown in Figure 2B indicated statistically significant reduction in break-point levels after l-THP administration (F3,26=18.34, p<0.001). Individual group comparisons using the Bonferroni t-test indicate a statistically significant reduction in break-point after 1 mg/kg (t=3.53, p<0.01), 3 mg/kg (t=2.97, p<0.05) or 10 mg/kg (t=7.39, p<0.001), when compared with the vehicle treatment group.
Figure 2.
Effect of l-THP on cocaine self-administration under PR reinforcement. Panel A shows representative records of an individual animal illustrating a reduction in the PR break-point for cocaine self-administration from 62 after vehicle (1 ml sterile water, i.p.; upper trace) to 32 after l-THP (3 mg/kg i.p., 30 min prior to test; lower trace) pretreatment. Each vertical line indicates a cocaine infusion (0.5 mg/kg/infusion). The number between the vertical lines indicates the work demand (progressively increased PR ratio, i.e., number of lever presses) for a subsequent cocaine infusion. The PR breakpoint was defined as the highest completed work requirement (lever-presses) to receive the last cocaine infusion. Panel B depicts the percent changes in break-point for cocaine self-administration after each dose of l-THP pretreatment. *p<0.05, ** p<0.01, *** p<0.001, when compared to the vehicle (0 mg/kg l-THP) pretreatment group.
3.3. Effects of l-THP on sucrose self-administration
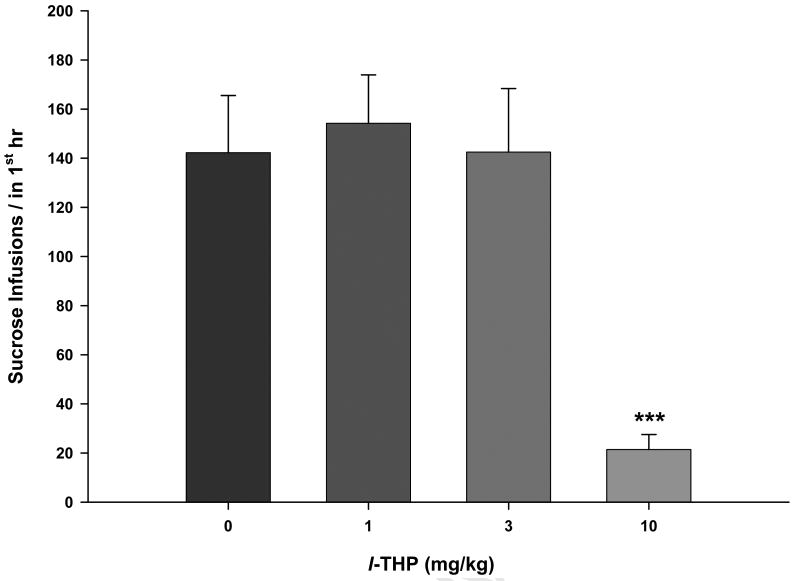
In contrast to the l-THP-induced alterations in cocaine self-administration under FR2 reinforcement, 1 or 3 mg/kg l-THP failed to, but 10 mg/kg l-THP significantly inhibited sucrose self-administration under FR2 reinforcement conditions (Fig. 3). One-way ANOVA for repeated measurements revealed a statistically significant l-THP treatment main effect (F3,23=11.36, p<0.001). Post-hoc individual group comparisons revealed a statistically significant reduction in sucrose self-administration after 10 mg/kg (t=4.62, p<0.001), but not after 1 mg/kg (t=0.78, p>0.05) or 3 mg/kg (t=0.21, p>0.05) l-THP administration.
Figure 3.
Effects of l-THP on oral sucrose self-administration under FR2 reinforcement, demonstrating that 10 mg/kg, but not 1 mg/kg or 3 mg/kg, l-THP inhibited sucrose self-administration behavior. ***p<0.001, when compared with the vehicle (0 mg/kg l-THP) control group.
3.4. Effects of l-THP on cocaine-enhanced brain stimulation reward
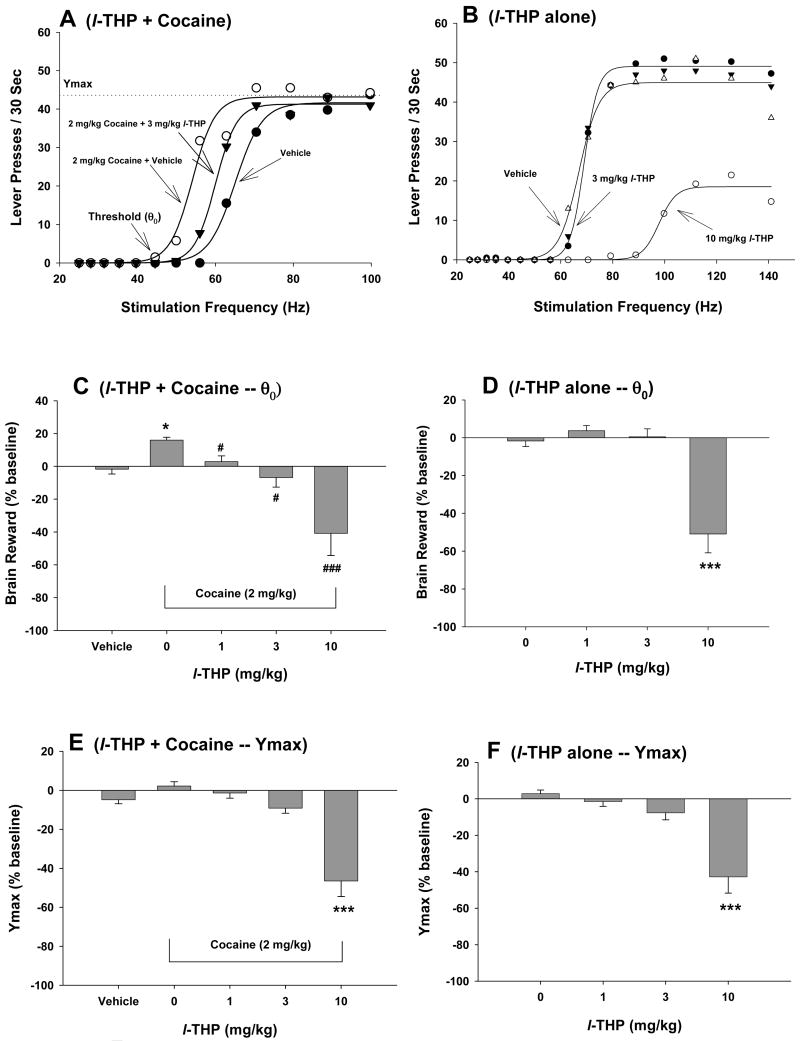
Figure 4A shows representative rate-frequency function curves for BSR, indicating the BSR threshold (θ0, Hz), the maximal operant response (Ymax, lever presses) produced by an animal for BSR, and the effects of cocaine on BSR in the presence of vehicle or l-THP. Systemic administration of cocaine (2 mg/kg) produced a significant enhancement in BSR, as indicated by the leftward shift in the rate-frequency function curve and lowered BSR threshold (θ0) (Fig. 4A). Pretreatment with l-THP (3 mg/kg, i.p., 30 min prior to test) significantly attenuated the cocaine-induced decrease in θ0 value (Fig. 4A). Figure 4B shows the effects of l-THP alone on BSR itself, indicating that 3 mg/kg l-THP has no significant effect on BSR, while 10 mg/kg l-THP significantly shifted the rate-frequency function curve to the right and also lowered the Ymax value. Figures 4C and 4D show the effects on l-THP on cocaine-enhanced BSR as assessed by the threshold (θ0), while Figures 4E and 4F show Ymax values. Cocaine (2 mg/kg) alone produced a significant enhancement of BSR (Figure 3C: vehicle versus 2 mg/kg cocaine, t=40.16, p<0.001), but had no effect on Ymax level (Figure 3E: vehicle versus 2 mg/kg cocaine, t=3.90, p=NS). Pretreatment with l-THP (1, 3, 10 mg/kg, i.p.) dose-dependently inhibited (and at 10 mg/kg reversed) the cocaine-enhanced BSR. One-way ANOVA with repeated measurements for the data shown in Fig. 4C (θ0 data, 0, 1, 3, 10 mg/kg l-THP) revealed a statistically significant treatment main effect (F3,19=34.62, p<0.001). Individual group comparisons revealed a statistically significant l-THP-induced reduction in cocaine-enhanced BSR after 1 mg/kg (t=1.92, p<0.05), 3 mg/kg (t=3.16, p<0.05), or 10 mg/kg l-THP (t=13.52, p<0.05). Indeed, at 10 mg/kg, l-THP produced a strikingly robust (∼40%) inhibition of brain reward. One-way ANOVA for repeated measurements performed on the data shown in Figure 4E (Ymax data, 0-10 mg/kg l-THP) revealed a statistically significant treatment main effect (F3,21=61.72, p<0.001). Individual group comparisons revealed a statistically significant reduction in Ymax values after 10 mg/kg (t=12.32, p<0.01), but not after 1 mg/kg (t=0.56, p=NS) or 3 mg/kg (t=1.72, p=NS) l-THP. At 10 mg/kg, l-THP alone significantly inhibited BSR (Figure 4D: F3,21=10.95, p<0.001; Bonferroni t=4.25, p<0.001) and lowered Ymax (Figure 4F: F3,21=12.34, p<0.001; Bonferroni t=5.31, p<0.001), but not 1 or 3 mg/kg.
Figure 4.
Effects of cocaine and/or l-THP on electrical brain stimulation reward (BSR). Panel A shows a representative rate-frequency function for BSR, indicating the BSR threshold (θ0) and Ymax. Cocaine (2 mg/kg, i.p.) shifted the rate-frequency function to the left, lowering the BSR θ0 threshold. Pretreatment with l-THP (3 mg/kg, i.p.) significantly attenuated the cocaine-induced decrease in BSR threshold (θ0). Panel B shows the effects of l-THP alone on BSR, indicating that 10 mg/kg, but not 3 mg/kg, l-THP significantly increased the threshold (θ0) (i.e., shifted the rate-response function to the right) and also lowered Ymax levels. Panel C shows the mean percentage changes in BSR threshold (θ0), indicating that l-THP (1-10 mg/kg, i.p.) dose-dependently inhibited 2 mg/kg cocaine-enhanced BSR. Panel D shows the mean percentage changes in BSR threshold (θ0) produced by l-THP alone, indicating that 10 mg/kg, but not 1 mg/kg or 3 mg/kg, l-THP inhibited BSR. Panel E shows the effects of l-THP and cocaine on BSR Ymax levels, indicating that 2 mg/kg cocaine had no effect on Ymax levels, but l-THP, at 10 mg/kg, but not 1 or 3 mg/kg, significantly lowered Ymax in the presence of cocaine. Panel F shows the effects of l-THP alone on BSR Ymax levels, indicating that l-THP, at 1 or 3 mg/kg, i.p. failed to, but 10 mg/kg of l-THP significantly lowered BSR Ymax levels. *p<0.05, ***p<0.001, when compared with the vehicle control group in each panel. # p<0.05, ### p<0.001, when compared with the cocaine only group in Panel C.
3.5. Effects of l-THP on locomotor activity
Figure 5A shows time courses of the effects of l-THP on locomotor activity, indicating that 3 mg/kg l-THP failed to, but 10 mg/kg l-THP significantly inhibited spontaneous locomotion in rats. Figure 5B shows the total counts (i.e., AUC) for locomotion after vehicle or l-THP. One-way ANOVA for repeated measurements revealed a statistically significant l-THP treatment main effect (F2,12=92.21, p<0.001). Individual group comparisons indicated a significant reduction in locomotion after 10 mg/kg (t=10.38, p<0.001), but not after 3 mg/kg (t=2.39, p=NS) l-THP.
3.6. Effects of l-THP on cocaine-enhanced DA in the NAc
Figures 6A and 6B show that l-THP (1, 3, 10 mg/kg i.p.) alone failed to alter NAc DA (Fig. 6A: F3,22=2.49, p=NS; Fig. 6B: F3,16=2.47, p=NS). Cocaine (10 mg/kg i.p.) produced a significant increase (∼400%) in NAc DA (Fig. 6C). L-THP pretreatment (1, 3, 10 mg/kg) dose-dependently augmented cocaine's enhancing effect on NAc DA (Figs. 6C, 6D). Two-way ANOVA for repeated measures over time for the data shown in Figure 6C revealed a statistically significant treatment main effect (F4,31=18.19, p<0.001), time main effect (F11,44=71.48, p<0.001) and treatment × time interaction (F44,341=7.67, p<0.001). Individual group comparisons revealed that the cocaine-induced increases in NAc DA were statistically significant, when compared with baselines in each group. The augmentation by l-THP of cocaine-enhanced NAc DA was also statistically significant, when compared with the cocaine treatment group, after 3 mg/kg (t=3.10, p<0.05) or 10 mg/kg (t=4.81, p<0.001), but not after 1 mg/kg (t=0.57, p=NS) l-THP (Fig. 6C). Similarly, one-way ANOVA for the data shown in Figure 6D revealed a statistically significant treatment main effect (F4,31=17.23, p<0.001). Individual group comparisons revealed that cocaine-induced increases in NAc DA were statistically significant (compared with the vehicle treatment group), and that the augmentation of cocaine-enhanced NAc DA was statistically significant, when compared with the cocaine treatment group, after 3 mg/kg (t=2.57, p<0.05) and 10 mg/kg (t=4.91, p<0.001), but not after 1 mg/kg (t=0.47, p=NS) l-THP.
3.7. Histology
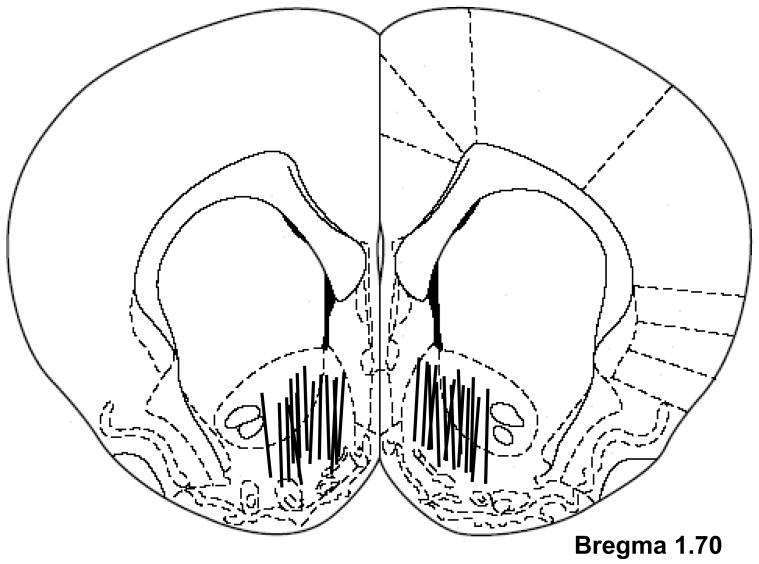
Figure 7 shows the locations of microdialysis probes in the NAc. The membrane portions of the probes were located in both the NAc shell and core, but predominantly in the shell.
Figure 7.
Diagram illustrating the placements of microdialysis probes within the NAc. The active microdialysis membrane portions were located within both the shell and core of the NAc.
4. Discussion
The present study demonstrates that systemic administration of l-THP (1, 3, 10 mg/kg) dose-dependently increased, while mg/kg decreased the rate of cocaine self-administration under FR reinforcement. The increase in cocaine self-administration produced by the lower l-THP doses is most likely a compensatory response to a reduction in cocaine's rewarding effects, because the same doses of l-THP dose-dependently inhibited cocaine self-administration under PR reinforcement and also inhibited cocaine-enhanced brain stimulation reward (BSR). In contrast to the effects on cocaine's actions, only the 10 mg/kg dose of l-THP (but not 1 or 3 mg/kg) produced any effect on sucrose self-administration and locomotion, suggesting a relatively selective antagonism of l-THP on cocaine's actions at lower l-THP doses. The in vivo microdialysis data suggest that a postsynaptic, but not presynaptic, DA receptor blockade mechanism may underlie the antagonism of l-THP on cocaine's rewarding effects.
Intravenous drug self-administration is the most commonly used animal model to evaluate a drug's rewarding effects (Gardner, 2000). There are two major self-administration paradigms, i.e. FR and PR reinforcement. Under FR reinforcement, the addictive drug is readily available to animals under low-effort (low work demand) and high reward (high dose of drug) conditions. In the present study, we found that low doses of l-THP increased, while the highest dose of l-THP inhibited, cocaine self-administration under FR2 reinforcement. This finding appears to conflict with a previous study demonstrating that l-THP produced a dose-dependent reduction in cocaine self-administration under FR4 reinforcement (Mantsch et al., 2007). This difference may be related to the different self-administration protocols used in the two studies. In the present experiment, we observed the effects of different doses of l-THP on cocaine self-administration at a single fixed cocaine dose (0.5 mg/kg/infusion) during a 3-hr self-administration duration. In contrast, Mantsch and colleagues used an alternating cocaine (30-min) and food (15-min) self-administration approach to observe the effects of l-THP on multiple doses of cocaine and food reinforcement. That is a very complex, rarely used approach in experimental animals, although it is often used in non-human primates. First, repeated food reward during cocaine self-administration has been shown to inhibit cocaine self-administration (de la Garza and Johnson, 1987; Comer et al., 1995). Thus, it is not surprising that l-THP appeared to be more potent or effective in antagonizing cocaine reward in the presence of food reward. Second, in that study, l-THP only inhibited cocaine self-administration maintained by the lower cocaine doses (0.031, 0.063, 0.125, 0.25 mg/kg/infusion), but not by the higher cocaine doses (0.5, 1.0 mg/kg/infusion). Indeed, at the highest cocaine self-administration dose (1 mg/kg/infusion), l-THP produced an increase in cocaine self-administration. This is consistent with our findings using a relatively highly rewarding dose (0.5 mg/kg/infusion) of cocaine. Third, animals tested under short time (30 min) and very low doses of cocaine may fail to reach a stable (maintenance) level of self-administration, which may prevent animals from compensatory responses to a change in drug reward after l-THP administration. Thus, both studies demonstrate that systemic administration of l-THP inhibits cocaine's rewarding effects. Animals may display an increase or decrease in cocaine self-administration, depending upon the strength of cocaine reward after l-THP administration.
In contrast to FR reinforcement, the PR self-administration paradigm appears to be more sensitive to changes in brain reward responses to drugs, as break-points are highly sensitive to variations in doses of drugs of abuse (Richardson and Roberts, 1996; Arnold and Roberts, 1997; Stafford et al, 1998). In the present study, we found that l-THP produced a dose-dependent reduction in PR break-points, suggesting a dose-dependent reduction in cocaine's rewarding effects. This is consistent with our finding above under FR reinforcement. In addition, motivation for drug-taking behavior is positively correlated with drug dose or reward strength (Arnold and Roberts, 1997; Stafford et al, 1998). Thus, the reduction in PR cocaine self-administration suggests that l-THP may also inhibit incentive motivational properties of cocaine.
Our conclusion with respect to l-THP's attenuation of cocaine's reward efficacy is further supported by our finding that l-THP also dose-dependently inhibited cocaine-enhanced BSR. The rewarding effects of brain stimulation are thought to involve the same reward circuits as, and to summate with, the rewarding effects of addictive drugs (Bauco and Wise, 1997). Cocaine significantly shifts the stimulation-response curve to the left or decreases BSR threshold, indicating summation of the reward produced by the electrical brain stimulation and the cocaine (Bauco and Wise, 1997). Such a reduction in both PR cocaine self-administration (above) and cocaine-enhanced BSR is unlikely due to non-specific sedation or locomotor inhibition, because the lower effective doses (1, 3 mg/kg) of l-THP did not alter locomotor activities, sucrose self-administration, or BSR Ymax, a parameter highly sensitive to locomotor inhibition. These data suggest that low doses of l-THP selectively inhibit cocaine's rewarding effects without sedation or locomotor inhibition.
However, when the dose was increased to 10 mg/kg, l-THP significantly inhibited locomotion, sucrose self-administration, and BSR itself. In addition, it also lowered BSR Ymax levels, suggesting that at 10 mg/kg, l-THP does produce sedation, locomotor inhibition and aversive effects in experimental animals, as reported previously (Jin, 1987; Jin et al., 2002; Mantsch et al., 2007). However, the same high dose (10 mg/kg) of l-THP increased cocaine self-administration under FR reinforcement, suggesting that the reduction in PR cocaine self-administration and cocaine-enhanced BSR is unlikely due to sedation or locomotor inhibition. Taken together, these findings from several different animal models suggest that high doses of l-THP do produce sedation or locomotor inhibition, but do not affect locomotor ability under conditions of high motivation. Animals can still perform motivation-driven drug-taking or drug-seeking behavior after l-THP administration (Jin, 1983; Liu et al., 2005).
The mechanisms by which l-THP inhibits drug's rewarding effects are not understood. It is well documented that cocaine, methamphetamine or opiates act on a common neural substrate, i.e., the mesolimbic DA projection pathway (Wise, 2005; Xi and Gardner, 2007) by different mechanisms. For example, cocaine elevates extracellular DA by blocking DA transporters, while methamphetamine-induced increase in DA is mediated predominantly by reversal of DA transporter (Wise, 2005). In contrast, opiate-induced activation of the mesolimbic DA system is indirectly mediated by inhibition of VTA GABAergic neurons, which subsequently disinhibits VTA DA neurons (Xi and Stein, 2002). Based on this, it was hypothesized that a DA-related mechanism may underlie the antagonism by l-THP of the actions of cocaine, methamphetamine or opioids as reported previously (Ren et al., 2000; Luo et al., 2003; Liu et al., 2005; Mantsch et al., 2007). Consistent with this DA-related hypothesis, in vitro binding assays demonstrate that l-THP acts as a non-selective D1 and D2 receptor antagonist (Jin, 1987; Xu et al., 1989; Guo et al., 1997; Mantsch et al., 2007), suggesting that l-THP's antagonism of drug's rewarding effects is most likely mediated by blockade of brain D1 and D2 receptors in vivo. This is further supported by evidence that both D1 and D2 receptors are critically involved in cocaine self-administration and BSR (Wise, 2005; Ikemoto et al., 1997), and that selective D1, D2 or non-selective D1/D2 receptor antagonists inhibit cocaine self-administration (Woolverton and Virus, 1989; Bergman et al., 1990; Caine and Koob, 1994; Soyka and De Vry, 2000), BSR (Ranaldi and Beninger, 1994) and cocaine-enhanced BSR (Kita et al., 1999). However, such effects produced by selective D1 or D2, or non-selective D1/D2 receptor antagonists occur mainly at doses that also inhibit other rewarding behaviors such as food consumption, or produce marked locomotor inhibition or extrapyramidal movements (Soyka and De Vry, 2000; Platt et al., 2002; Gorelick et al., 2004). In contrast, l-THP, a natural non-selective DA receptor antagonist extracted from Chinese herbs, while producing significant sedation, produces no such unwanted side-effects as inhibition of natural reward, gross locomotion deficits or abnormal extrapyramidal movements (Jin, 1987; Jin et al., 2002). The mechanisms underlying such differences between l-THP and other DA receptor antagonists are unclear. One possibility is that selective blockade of one or two DA receptor subtypes leads to neuroadaptations of or increased DA binding to other DA receptors (Canales and Graybiel, 2000; Stanwood et al., 2000), which may subsequently attenuate the therapeutic effects or produce unwanted side-effects. In addition, the binding and actions of l-THP on other DA (in particular, D3), norepinephrine and 5-HT receptors (Mantsch et al., 2007), in addition to blockade of D1 and D2 receptors, may produce additive or synergistic therapeutic effects, while inhibiting unwanted side-effects.
To further determine whether a presynaptic or postsynaptic DA receptor mechanism underlies l-THP's actions, we observed the effects of l-THP on basal or cocaine-augmented NAc DA. We found that pretreatment with l-THP (1, 3, 10 mg/kg) augmented cocaine-enhanced NAc extracellular DA, similar to selective D2 or D3 receptor antagonists (Rougé-Pont et al., 2002; Xi and Gardner, 2007), suggesting an effect mediated by blockade of presynaptic D2 and/or D3 receptors. This is consistent with a previous report that l-THP potentiates medial forebrain bundle stimulation-induced increases in DA and attenuates apomorphine (a non-selective DA receptor agonist)-induced decreases in DA release in the dorsal striatum (Marcenac et al., 1986). Clearly, this augmentation in NAc extracellular DA mediated by blockade of presynaptic D2/D3 receptors cannot explain the antagonism by l-THP of cocaine's rewarding effects observed in the present study. Thus, a postsynaptic, rather than presynaptic, DA receptor antagonism appears more likely to mediate l-THP's anti-cocaine effects, in line with previous suggestions (Marcenac et al., 1986; Jin, 1987). In addition, we also found that the same doses of l-THP only modestly elevate extracellular NAc DA, suggesting that DA tone on presynaptic D2 and/or D3 receptors is lower in the absence of cocaine than that after cocaine. Given that cocaine addicts have been postulated to display a hypodopaminergic trait or a reward deficiency syndrome (Kuhar and Pilotte, 1996; Gardner, 1999), l-THP-induced modest increases in NAc DA could also contribute to its putative therapeutic effects by normalization of the postulated hypofunctional DA transmission in the NAc.
In conclusion, l-THP is a purified active ingredient from the Chinese herb Stephanie. Due to its marked sedative-analgesic action (Jin, 1987), l-THP has been used in China for more than 40 years for relieving pain and anxious insomnia. Given its non-selective DA receptor antagonist properties with similar affinity for D1, D2 and D3 receptors and its lessened liability for extrapyramidal movement side-effects, l-THP and its analogs have undergone clinical trials as potential anti-psychotic medications (Jin, et al., 2002; Ellenbroek et al., 2006). In the present study, we demonstrate that low dose l-THP also significantly and selectively inhibits cocaine's rewarding effects, supporting a potential use of l-THP, a traditional Chinese medicine, in the treatment of cocaine addiction.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, and by Chinese Ministry of Science and Technology grant number 2003CB51540 to Professor Zhang Yang.
Footnotes
Disclosure/Conflict of Interest: All authors hereby declare that, except for income received from their respective primary employers, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional services. There are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold JM, Roberts DCS. A critique of fixed ratio and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Synergistic effects of cocaine with lateral hypothalamic brain stimulation reward: lack of tolerance or sensitization. J Pharmacol Exp Ther. 1997;283:1160–1167. [PubMed] [Google Scholar]
- Bergman J, Kamien JB, Spealman RD. Antagonism of cocaine self-administration by selective dopamine D1 and D2 antagonists. Behav Pharmacol. 1990;1:355–363. doi: 10.1097/00008877-199000140-00009. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther. 1994;270:209–218. [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. Patterns of gene expression and behavior induced by chronic dopamine treatments. Ann Neurol. 2000;47(Suppl 1):S53–S59. [PubMed] [Google Scholar]
- Comer SD, Turner DM, Carroll ME. Effects of food deprivation on cocaine base smoking in rhesus monkeys. Psychopharmacology. 1995;119:127–132. doi: 10.1007/BF02246152. [DOI] [PubMed] [Google Scholar]
- Coulombe D, Miliaressis E. Fitting intracranial self-stimulation data with growth models. Behav Neurosci. 1987;101:209–214. doi: 10.1037//0735-7044.101.2.209. [DOI] [PubMed] [Google Scholar]
- de la Garza R, Johnson CE. The effects of food deprivation on the self-administration of psychoactive drugs. Drug Alcohol Depend. 1987;19:17–27. doi: 10.1016/0376-8716(87)90083-4. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Zhang XX, Jin GZ. Effects of (-)stepholidine in animal models for schizophrenia. Acta Pharmacol Sin. 2006;27:1111–1118. doi: 10.1111/j.1745-7254.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- Gardner EL. The neurobiology and genetics of addiction: implications of “reward deficiency syndrome” for therapeutic strategies in chemical dependency. In: Elster J, editor. Addiction: Entries and Exits. New York: Russell Sage Foundation; 1999. pp. 57–119. [Google Scholar]
- Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- Ge XQ, Zhang HQ, Zhou HZ, Xu ZX, Bian CP. Experimental studies with tetrahydropalmatine analogs in relieving morphine withdrawal syndromes. Chinese J Drug Depend. 1999;8:108–112. [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Guo X, Wang LM, Liu J, Jin GZ. Characteristics of tetrahydroprotoberberines on dopamine D1 and D2 receptors in calf striatum. Acta Pharmacologica Sinica. 1997;18:225–230. [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology. 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci. 1997;17:8580–8587. doi: 10.1523/JNEUROSCI.17-21-08580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin GZ. (-)-Tetrahydropalmatine and its analogues as new dopamine receptor antagonists. Trends Pharmacol Sci. 1987;8:81–82. [Google Scholar]
- Jin GZ, Zhu ZT, Fu Y. (-)-Stepholidine: a potential novel antipsychotic drug with dual D1 receptor agonist and D2 receptor antagonist actions. Trends Pharmacol Sci. 2002;23:4–7. doi: 10.1016/s0165-6147(00)01929-5. [DOI] [PubMed] [Google Scholar]
- Jin WQ, Zhang HP, Chen XJ, Chi ZQ. Effects of rotundine on morphine tolerance and dependence. Chinese Periodical. 1998;4:1136–1142. [Google Scholar]
- Kita K, Shiratani T, Takenouchi K, Fuzazako H, Takigawa M. Effects of D1 and D2 dopamine receptor antagonists on cocaine-induced self-stimulation and locomotor activity in rats. Eur Neuropsychopharmacol. 1999;9:1–7. doi: 10.1016/s0924-977x(97)00098-9. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Pilotte NS. Neurochemical changes in cocaine withdrawal. Trends Pharmacol Sci. 1996;17:260–264. doi: 10.1016/0165-6147(96)10024-9. [DOI] [PubMed] [Google Scholar]
- Liu YL, Liang JH, Yan LD, Su RB, Wu CF, Gong ZH. Effects of l-tetrahydropalmatine on locomotor sensitization to oxycodone in mice. Acta Pharmacol Sin. 2005;26:533–538. doi: 10.1111/j.1745-7254.2005.00101.x. [DOI] [PubMed] [Google Scholar]
- Luo JY, Ren YH, Zhu R, Lin DQ, Zheng JW. The effect of l-tetrahydropalmatine on cocaine induced conditioned place preference. Chin J Drug Depend. 2003;12:177–179. [Google Scholar]
- Mantsch JR, Li SJ, Risinger R, Awad S, Katz E, Baker DA, Yang Z. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology. 2007;192:581–591. doi: 10.1007/s00213-007-0754-7. [DOI] [PubMed] [Google Scholar]
- Marcenac F, Jin GZ, Gonon F. Effect of l-tetrahydropalmatine on dopamine release and metabolism in the rat striatum. Psychopharmacology. 1986;89:89–93. doi: 10.1007/BF00175196. [DOI] [PubMed] [Google Scholar]
- Nann-Vernotica E, Donny EC, Bigelow GE, Walsh SL. Repeated administration of the D1/5 antagonist ecopipam fails to attenuate the subjective effects of cocaine. Psychopharmacology. 2001;155:338–347. doi: 10.1007/s002130100724. [DOI] [PubMed] [Google Scholar]
- Newton TF, Ling W, Kalechstein AD, Uslaner J, Tervo K. Risperidone pre-treatment reduces the euphoric effects of experimentally administered cocaine. Psychiatry Res. 2001;102:227–233. doi: 10.1016/s0165-1781(01)00255-4. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology. 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Beninger RJ. The effects of systemic and intracerebral injections of D1 and D2 agonists on brain stimulation reward. Brain Res. 1994;651:283–292. doi: 10.1016/0006-8993(94)90708-0. [DOI] [PubMed] [Google Scholar]
- Ren YH, Zhu Y, Jin GZ, Zheng JW. Levo-tetrahydropalmatine inhibits the expression of methamphetamine-induced conditioned place preference in rats. Chinese J Drug Depend. 2000;9:182–186. [Google Scholar]
- Rothman RB, Glowa JR. A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development. Focus on GBR 12909. Mol Neurobiol. 1995;11:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J Neurosci. 2002;22:3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer MA, Kumor KM, Jaffe JH. Effects of intravenous cocaine are partially attenuated by haloperidol. Psychiatry Res. 1989;27:117–125. doi: 10.1016/0165-1781(89)90127-3. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Soyka M, De Vry J. Flupenthixol as a potential pharmacotreatment of alcohol and cocaine abuse/dependence. Eur Neuropsychopharmacol. 2000;10:325–332. doi: 10.1016/s0924-977x(00)00088-2. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Lucki I, McGonigle P. Differential regulation of dopamine D2 and D3 receptors by chronic drug treatments. J Pharmacol Exp Ther. 2000;295:1232–1240. [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav. 1989;32:691–697. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:1–21. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. GABAergic mechanisms of opiate reinforcement. Alcohol Alcohol. 2002;37:485–494. doi: 10.1093/alcalc/37.5.485. [DOI] [PubMed] [Google Scholar]
- Xu SX, Yu LP, Han YP, Chen Y, Jin GZ. Effects of tetrahydroprotoberberines on dopamine receptor subtypes in brain. Acta Pharmacologica Sinica. 1989;10:104–110. [PubMed] [Google Scholar]
- Yang Z, Chen H, Hao W, Jin GZ, Li SJ. Medication of l-tetrahydropalmatine significantly increased the abstinence rate in heroin addicts. 68th Annual Scientific Meeting of the College on Problems of Drug Dependence; Scottsdale, AZ. 2006. Abstract #109. [Google Scholar]