Orthotopic liver transplantation offers an extraordinary approach to end-stage liver disease by eliminating pathology and restoring physiology to normal, rather than by the traditional approach of merely controlling its life-threatening complications.
Between March, 1980 and December, 1985 500 patients, including 203 children and 297 adults, with end-stage liver disease have been treated with orthotopic liver transplantation at the University of Colorado and the University of Pittsburgh, using cyclosporine and low-dose prednisone. Three hundred forty patients (68 per cent) are surviving 1 month to 6 years after transplantation.
GENERAL INDICATIONS
Candidates for liver transplantation must have an irreversible disease for which conventional medical and surgical therapy have been exhausted. In most cases this is a disease of the liver such as chronic aggressive hepatitis or biliary atresia that leads to end-stage cirrhosis. 22 However, liver transplantation also has been performed to correct inborn errors of liver metabolism which result mainly in pathology in other organ systems. 26 Two years ago the first successful simultaneous heart and liver transplant was performed in Pittsburgh to correct a disorder of cholesterol metabolism in a 6-year-old girl with severe atherosclerotic heart disease.19 The heart transplant was necessary to repair the irreparable damage to the girl’s heart. The liver transplant was necessary to correct the genetic defect responsible for the disease. One year ago, a 17-year-old boy with hemophilia underwent liver transplantation for chronic aggressive hepatitis acquired as a complication of factor VIII transfusions. 10 The operation restored both his liver function and coagulopathy to normal. Although we have not yet reached the point of recommending liver replacement for patients with this form of hemophilia, the risks and limitations of factor VIII replacement therapy are significant and such a consideration may be appropriate in the future.
The timing of liver transplantation is important. With progression of disease, the patient’s natural resources are gradually exhausted until the final uncontrollable downhill course when the remaining liver function is inadequate to maintain adequate homeostasis. Transplantation is best performed before this last critical period. This allows the patient to undergo surgery while still an acceptable risk but avoids premature operation and immunosuppression. A crisis can be precipitated, however, during the interim period by conditions in which increased demands are placed on the already compromised liver. These conditions include infection, trauma, hemorrhage, and dehydration. Complications of liver disease may force earlier intervention, either because they are refractory to conventional therapy or because application of appropriate therapy will prevent or seriously interfere with the performance of orthotopic liver transplantation.
PORTAL HYPERTENSION AND UPPER GASTROINTESTINAL BLEEDING
Orthotopic liver transplantation provides a perfect transcapillary porta caval shunt through the new liver, and will effectively stop upper gastrointestinal bleeding secondary to portal hypertension. Temporizing measures including sclerotherapy and medical treatment are useful until orthotopic liver transplantation can be performed. Shunting procedures such as portosystemic and central splenorenal bypass make orthotopic liver transplantation much more difficult and should be avoided. Our experience with selective distal splenorenal shunts2 has been more favorable because the surgical manipulation is at a distance from the hilum. The condition of the portal vein is generally satisfactory after distal splenorenal shunt and the shunt, if still patent, can usually be dealt with by splenectomy at the time of transplantation.2 Splenectomy, either in association with shunting or alone, may be harmful because it is associated with a Significant incidence of portal vein thrombosis.2
ASCITES
Ascites by itself is not an indication for liver replacement but is an indicator of cardiovascular instability and advancing portal hypertension. Severe intractable ascites, often associated with pleural effusion, spontaneous bacterial peritonitis, and anasarca, clearly indicates that the patient has entered the final phase of end-stage liver disease and needs to be transplanted.
Peritoneovenous shunts do not significantly complicate orthotopic liver transplantation and can be used when indicated. The shunt must be interrupted at the start of the transplant procedure to prevent air embolism.
HEPATIC COMA
Hepatic encephalopathy is an extremely serious manifestation of end-stage liver disease and significantly alters patient prognosis. Patients with low-grade encephalopathy often do well after transplantation, but the mortality of patients with stage IV coma is high. Nevertheless, a successful liver transplant can reverse all but the most severe degrees of hepatic encephalopathy, and even patients in stage IV coma have completely reversed after transplantation. Terminal hepatic encephalopathy, manifesting as brain death, will not be reversed and is an absolute contraindication to orthotopic liver transplantation.
HEPATIC RICKETS
Hepatic rickets is frequently seen with chronic liver failure and is incapacitating.25 This disorder is reversible after orthotopic liver transplantation, but if it has been allowed to progress to an advanced degree, rehabilitation is slow and difficult. We recommend that transplantation be performed before advanced osteoporosis has set in.
SPECIFIC INDICATIONS
Disease entities that have been primary indications for orthotopic liver transplantation are shown in Table 1.
Table 1.
Primary Indications for Liver Transplantation
| INDICATION | PEDIATRIC (N = 203) | ADULT (N = 297) | TOTAL (N = 500) |
|---|---|---|---|
| Cirrhosis | 21 (10.3) | 99 (33.3) | 120 (24.0) |
| Primary biliary cirrhosis | 0 (0.0) | 83 (28.0) | 83 (16.6) |
| Biliary atresia | 99 (48.8) | 0 (0.0) | 99 (19.8) |
| Inborn errors of metabolism | 45 (22.2) | 23 (9.7) | 68 (13.6) |
| Sclerosing cholangitis | 2 (1.0) | 41 (13.8) | 43 (8.6) |
| Primary liver tumors | 0 (0.0) | 20 (6.7) | 20 (4.0) |
| Secondary biliary cirrhosis | 3 (1.5) | 12 (4.0) | 15 (3.0) |
| Familial cholestasis | 14 (6.9) | 0 (0.0) | 14 (2.8) |
| Acute hepatic necrosis | 5 (2.5) | 6 (2.0) | 11 (2.2) |
| Budd-Chiari syndrome | 1 (0.5) | 7 (2.4) | 8 (1.6) |
| Neonatal hepatitis | 6 (3.0) | 0(0.0) | 6 (1.2) |
| Others | 7 (3.5) | 6 (2.0) | 13 (2.6) |
Cirrhosis, which usually is secondary to chronic aggressive hepatitis, is the most common indication for liver transplantation. Patients with surface and core antigen-positive hepatitis B are at high risk of recurrence after transplantation. The series also includes small numbers of patients with cryptogenic cirrhosis and alcoholic cirrhosis. Alcoholic cirrhosis has been considered historically as a poor indication for orthotopic liver transplantation because of poor patient compliance and poor patient medical condition. However, there have been outstanding exceptions and each case has to he examined on its individual merits.
Biliary atresia is the second most common indication for liver transplantation and accounts for nearly 50 per cent of the pediatric cases.3 Portoenterostomy can be useful in allowing time for growth and development of infants, but most patients with biliary atresia will ultimately require transplantation. Multiple revisions of portoenterostomies and creation of external stomas can significantly complicate transplantation and should be avoided.
Primary biliary cirrhosis, an idiopathic, indolent disease most often affecting middle aged women, is the third most common indication. Transplantation is indicated when bilirubin begins to show an increase in the rate of rise or exceeds 10 to 15 mg/dl, or in the prevention of progressive hepatic rickets.
Inborn errors of metabolism, including Wilson’s disease, alpha-1-antitrypsin deficiency, tyrosinemia, glycogen storage disease, and hemochromatosis, account for 13.6 per cent of the current series. As mentioned, hypercholesterolemia and hemophilia also have been corrected by liver replacement.10, 19
During the past 2 years more cases of sclerosing cholangitis have been accepted for transplantation. Many of these patients have had previous surgical procedures involving the hepatic hilum. The current emphasis on percutaneous radiologically guided intervention should minimize the need for open surgical procedures at the hilum before transplantation. Coexisting ulcerative colitis is often burned out before transplantation is necessary. However, if a colectomy is indicated, it is much better tolerated after liver transplantation rather than vice versa. The existence of an ileostomy during orthotopic liver transplantation predisposes to infection and is a major hazard.
Secondary biliary cirrhosis can be corrected by orthotopic liver transplantation. These cases are often technically demanding because of the multitude of previous procedures in the hepatic hilum.
Budd-Chiari syndrome can be effectively treated by transplantation.12 Preoperative evaluation of the portal vein and inferior vena cava is crucial because of the high incidence of thrombosis in these structures. Extensive venous thrombectomy may be possible at surgery. After transplantation, it is essential that these patients be maintained on moderate anticoagulation (prothrombin time 1.5 to 2 times normal).
Primary liver tumors are considered poor indications for orthotopic liver transplantation because of a high incidence of fatal recurrence after transplantation. We have not considered metastatic liver tumors as indications for orthotopic liver transplantation, with the exception of metastatic carcinoid tumor, which has a benign biologic behavior. There have been rare examples of orthotopic liver transplantation performed by other centers for metastatic cancer.11
Evaluation
The preoperative evaluation must asssess advisability, feasibility, and the possible need for multiple organ transplantation.
Advisability
The history and physical examination remain the most accurate means of making this assessment in most cases. In doubtful cases, liver volume, liver chemistries, ICG clearance, and/or a liver biopsy may be helpful.
Feasibility
Orthotopic liver transplantation may not be feasible for technical reasons or because of pathology involving other organs. Portal vein thrombosis was once considered an absolute contraindication to orthotopic liver transplantation. However, the use of venous grafts has allowed successful transplantation in some cases, but at a considerable risk.15 Doppler ultrasound is useful as a screening test for assessment of portal vein patency.8 In complex cases angiography is required.
The clinical condition of the patient may necessitate a careful evaluation of the cardiorespiratory and renal systems to determine the ability of the patient to tolerate the stress of orthotopic liver transplantation, including the potential nephrotoxicity of cyclosporine. Extra-hepatic infection and metastatic disease are absolute contraindications to orthotopic liver transplantation.
Need for Additional Organ Transplants
Orthotopic liver transplantation can be expected to have satisfactory results only if all pathophysiology is related to liver failure. If extra-hepatic organs are involved, a determination has to be made as to whether or not 1) the patient has enough reserve to survive the perioperative period, and if not, if there are satisfactory artificial means to sustain the patient and 2) the process is reversible after successful orthotopic liver transplantation.
Significant experience has been acquired with multiple organ transplants, especially simultaneous liver and kidney transplantation. Many patients with chronic liver failure have compromised renal function, either as a consequence of liver disease or in addition to it. The stress of surgery and the nephrotoxicity of cyclosporine may further compromise renal function after transplantation. Most patients with renal dysfunction secondary to chronic liver failure can recover adequate renal function after liver replacement, but patients with intrinsic renal disease may not and are possible candidates for simultaneous transplantation. The time of the orthotopic liver transplantation may offer an “immunologic window” for a simultaneous kidney transplant. It has been shown that a simultaneous kidney transplant may be successfully performed even in the face of a positive donor lymphocytotoxic crossmatch.4 After orthotopic liver transplantation, patients may develop high antibody titers and later it may be difficult to find a biocompatible kidney allograft. Demonstration of irreversible pathology (polycystic, small, or shrunken kidneys), or knowledge that the kidneys were normal before the liver decompensated facilitate decision making. The remaining cases require skillful clinical judgment; objective criteria are being developed as experience grows.
THE TRANSPLANT COMMITTEE
After the evaluation is completed, the candidates are presented to a transplantation committee including surgeons, hepatologists, nurses, social service personnel, and immunopathologists. The committee makes the final decision to activate the patient on the transplant waiting list and makes an assessment of priority.
DONOR MATCHING
When a liver donor becomes available, he or she has to be matched for liver size and ABO blood group with a recipient. Orthotopic liver transplantation across ABO blood groups can be done without risk of hyperacute rejection and with good graft survival.5 However, graft survival for ABO identical grafts is better than that for ABO compatible but nonidentical or ABO incompatible grafts. We therefore limit ABO nonidentical or incompatible grafts to patients such as small children for whom the supply of available donors is severely limited or for patients in urgent need of transplantation or retransplantation.
Size discrepancies are more problematic. Generally speaking a small liver can be fitted into a larger cavity, but the reverse adjustment is difficult. Successful transplantations have been performed after resection of parts of a graft,1 but this is done at the risk of additional complications and has only been used as a measure of last resort.
Selection of recipients of appropriate blood group and size to receive a liver graft is done using a point system which is based mainly on urgency of need, logistic considerations such as transportation requirements, and waiting time. HLA matching cannot be performed prospectively due to the time requirements of currently available techniques of liver preservation, and antibody crossmatching is unnecessary.
TRANSPLANTATION
Once the donor team is ready to be dispatched to obtain the graft, a recipient team is organized and anesthesia staff, the operating room personnel, the blood bank, and the intensive care unit are immediately notified. It is essential that the donor team maintain communication with the recipient team in order to coordinate the process of procurement and implantation.
The donor and recipient procedures have been described elsewhere18, 21 in detail and only a few points of particular interest will be mentioned here. The arterial blood supply to the liver is quite variable. A single hepatic artery originating from the celiac axis is only found about half the time. Left lateral segment branches from the left gastric artery and right lobar arteries from the superior mesenteric artery are common. Great care must be taken during procurement to preserve variant arteries. Aortoiliac allografts are taken from the donor for use in complicated reconstructions.
The recipient procedure consists of four stages: hepatectomy, revascularization, hemostasis, and biliary reconstruction. A major development in the recipient procedure has been the use of a venovenous bypass. 7 This procedure facilitates hepatectomy and maintains cardiovascular stability during the anhepatic phase of surgery. 17 The use of vascular grafts permits rearterialization of the allograft in complicated cases. 16 Iliac grafts routinely harvested from the donor can be used as aortohepatic grafts, between the recipient infrarenal aorta and the donor hepatic artery, if the recipient hepatic artery is inadequate (Fig. 1A and B). If the donor organ has a double arterial supply from the celiac axis and superior mesenteric artery, the origin of the celiac axis and superior mesenteric artery are sewn together using a fold-over Carrel patch technique. 6 The distal end of the superior mesenteric artery is then used as the point of anastomosis to the recipient (Fig. 2). Venous grafts (donor vena cava or iliac vein) can be used to replace a fibrotic or cavernomatous recipient portal vein15 or to add length to short vena cava cuffs. Whenever possible, the biliary tract is reconstructed by a duct to duct anastomosis over a T-tube stent. 18, 21 If this is not possible, the preferred anastomosis is a choledochojejunostomy with an internal stent to a Roux-en-Y jejunal limb (Fig 3). 18, 21
Figure 1.
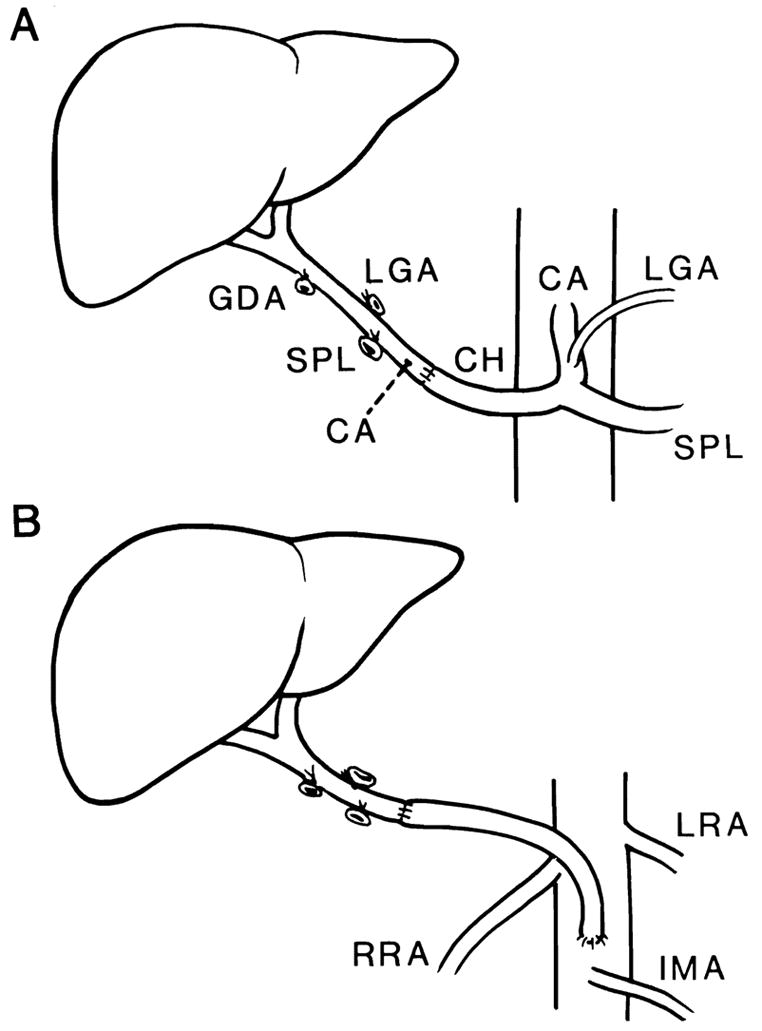
Methods of arterial reconstruction with a single hepatic arterial supply. A, Conventional arterial reconstruction by anastomosis of the donor hepatic artery to the recipient hepatic artery. The recipient gastroduodenal artery (not shown) has been divided. B, Alternative method of arterial reconstruction when recipient hepatic artery cannot be used. An arterial homograft from the infrarenal aorta is tunneled under the pancreas and duodenum and anastomosed to the donor hepatic artery.
Figure 2.
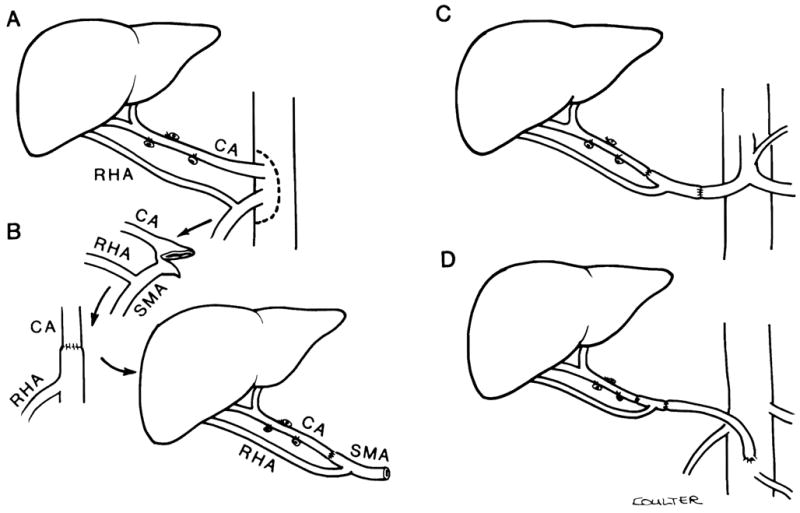
Methods of arterial reconstruction with complex hepatic arterial supply. A, A dual arterial supply to the liver with left hepatic artery originating from the celiac axis (CA) and right hepatic artery originating from the superior mesenteric artery (SMA) is preserved by resecting an aortic patch containing the origins of both CA and SMA. A segment proximal to the SMA, including the origin of the right hepatic artery, must also be preserved. B, The aortic patch is folded over to anastomose the origin of the CA to the SMA. The SMA distal to the origin of the right hepatic artery is then used for anastomosis to the donor hepatic artery. C, An alternative method of reconstruction using an interposition graft of donor SMA containing the origin of the right hepatic artery between the donor left hepatic and recipient common hepatic arteries. D, Use of a homograft from the infrarenal aorta in combination with the reconstruction in C when there is no suitable recipient hepatic artery available for anastomosis.
Figure 3.
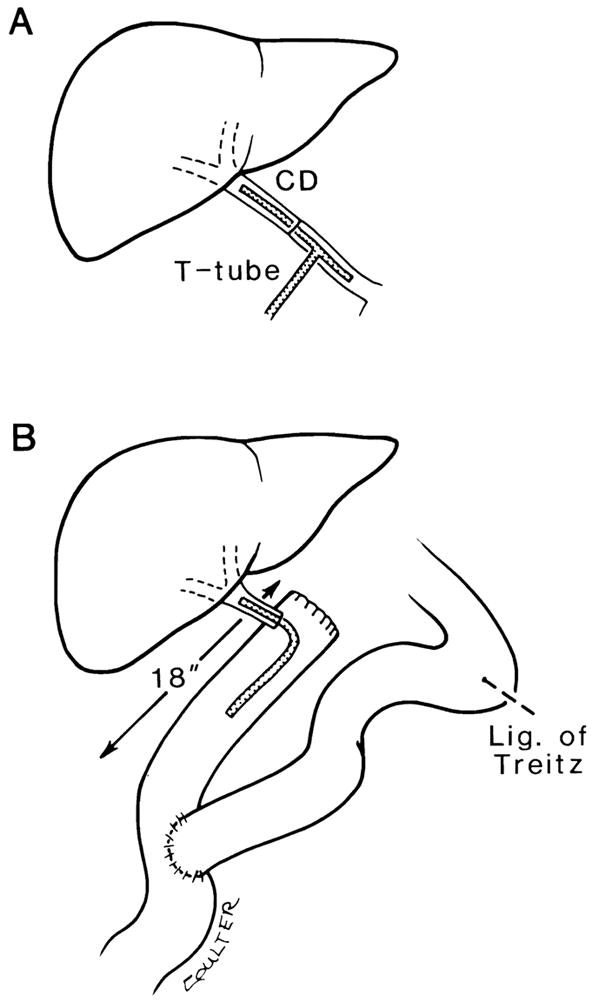
Biliary tract reconstruction. A, Direct duct to duct repair over a T-tube brought out through a stab incision in the recipient common duct contralateral to nearby vascular anastomoses. B, Biliarv reconstruction by end-to-side anastomosis of the donor Common bile duct to an 18 inch Roux-en-Y limb of proximal jejunum. A polyethylene infant feeding tube is used as an internal stent.
POSTOPERATIVE COURSE
The stage for the postoperative course is set by the preoperative status of the recipient, the quality of the donor organ, and the course of the recipient operation. Immunologic reactions and opportunistic infections can alter the process.
Potential technical complications are numerous. The most frequent are arterial thrombosis and biliary complications. Hepatic artery thrombosis is a potentially fatal complication which is much more frequent in children than in adults (10.4 versus 3.4 per cent). 24 It may result in gangrene of the liver or irreparable complications of ischemic necrosis of the donor bile duct. An occasional patient has survived without retransplantation because of the development of sufficient collateral arterial circulation. An anticoagulation protocol involving the use of low molecular weight dextran, low-dose heparin, and aspirin is currently under investigation. Early results indicate that this regimen may be effective in lowering the incidence of hepatic artery thrombosis.
Biliary complications, once called the Achilles’ heel of orthotopic liver transplantation, occurred in 52 (16.6 per cent) of 313 consecutive cases recently reviewed by Lerut et al. 9 The prognosis of patients with biliary tract complications has improved because earlier detection is possible with the current radiologic techniques. Ultrasound, CT scans, and T-tube cholangiograms are common baseline studies. Percutaneous cholangiograms and arteriograms are obtained when indicated. Primary biliary tract complications must be differentiated from bile leaks secondary to hepatic artery thrombosis.
The immunologic reactions after liver transplantation include rejection (acute and chronic) and graft versus host reactions. Acute or cellular rejection has protean manifestations, but under cyclosporine and prednisone usually presents with malaise, fever, and changes in the quality and quantity of bile. The diagnosis can be further suspected by an elevation of the serum bilirubin and hepatic transaminases and can be confirmed with a percutaneous liver biopsy. Cyclosporine in combination with low-dose steroids is to date the most effective known means of preventing rejection. Steroid boluses have been the preferred treatment of acute rejection. During the past 2 years, a new form of antilymphocyte antibody therapy, OKT-3 monoclonal antibody, has been used with satisfactory results. 20
The use of these agents requires a great deal of clinical judgment: insufficient use will result in graft loss due to rejection; overusage will result in opportunistic infections, which can be fatal. The matter is further complicated at times by the coexistence of rejection and opportunistic infection.
Lymphoproliferative disorders are considered manifestations of overimmunosuppression, especially with cyclosporine. 23 They usually will regress with reduction or withdrawal of immunosuppression.
Chronic rejection is a much more insidious process, which manifests as bile duct disappearance and fibrosis, is refractory to therapy, and requires retransplantation. The graft versus host reaction usually occurs in orthotopic liver transplantations across the ABO blood barrier, and usually presents as a combination of hemolytic anemia, fever, and thrombocytopenia, usually 2 to 3 weeks after operation. 13 Gastrointestinal symptoms are not unusual, but pneumonitis or severe rash is uncommon. It is usually a self-limiting syndrome and requires only supportive treatment.
RESULTS
Three hundred forty (68 per cent) of the 500 patients transplanted with cyclosporine and prednisone are alive, including 197 (66.3 per cent) of the adults and 143 (70.4 per cent) of the children.
The goal of orthotopic liver transplantation is restoration of a normal life, and this is achieved in the vast majority of successful orthotopic liver transplantation recipients. Eighty-one long-term survivors transplanted with cyclosporine–prednisone were reviewed by Shaw et al. 14 Only seven of these patients were disabled, and two additional patients were unemployed. The remainder were fully employed, active homemakers, or attending school. As the results of orthotopic liver transplantation are continuously improving, we are seeing increasing numbers of successful orthotopic liver transplant recipients among the productive individuals of our society.
Acknowledgments
This study was supported by Research Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Bismuth H, Houssin D. Partial resection of liver grafts for orthotopic or heterotopic liver transplantation. Trans Proc. 1985;17:279. [Google Scholar]
- 2.Esquivel CO, Klintmalm G, Iwatsuki S, et al. Liver transplantation in patients with patent splenorenal shunts. Surgery. (submitted) [PMC free article] [PubMed] [Google Scholar]
- 3.Esquivel CO, Starzl TE. In: Liver transplantation for biliary atresia. Glassman J, editor. Biliary Surgery; [Google Scholar]
- 4.Gordon RD, Fung JJ, Markus B, et al. The antibody crossmatch in liver transplantation. Surgery. (in press) [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon RD, Iwatsuki S, Esquivel CO, et al. Liver transplantation across ABO blood groups. Surgery. (in press) [PubMed] [Google Scholar]
- 6.Gordon RD, Shaw BW, Iwatsuki S, et al. A Simplified technique for revascularization of liver homografts with a variant right hepatic artery from the superior mesenteric artery. Surg Gynecol Obstet. 1985;160:474–476. [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith BP, Shaw BW, Jr, Hardesty RL, et al. Venovenous bypass without systemic anticoagulation for transplantation of the human liver. Surg Gynecol Obstet. 1985;160:270–272. [PMC free article] [PubMed] [Google Scholar]
- 8.Hagler NG, III, Bowne A, Skolnick ML, et al. Ultrasound determination of portal vein patency in patients being evaluated for possible orthotopic liver transplantation. Radiology. (in press) [Google Scholar]
- 9.Lerut J, Gordon RD, Iwatsuki S, et al. Biliary tract complications in human orthotopic liver transplantation. Transplantation. doi: 10.1097/00007890-198701000-00011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JG, Bontempo FA, Spero JA, et al. Liver transplantation in a hemophiliac. (Letter to the Editor) N Engl J Med. 1985;312:1189. doi: 10.1056/NEJM198505023121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margreiter R, Huber C, Niederwieser D, et al. Combined bone marrow and liver transplantation with total body irradiation and high-dose cyclophosphamide in the treatment of metastatic liver disease. Trans Proc. 1985;17:296. [PubMed] [Google Scholar]
- 12.Putman CW, Porter KA, Weil R, III, et al. Liver transplantation for the Budd-Chiari syndrome. JAMA. 1976;236:1142–1143. [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsey G, Nusbacher J, Starzl TE, et al. Isohemagglutinins of graft origin after ABO-unmatched liver transplantation. N Engl J Med. 1984;311:1167–1170. doi: 10.1056/NEJM198411013111807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw BW, Gordon RD, Iwatsuki S. Transplantation of the liver. Surgical Treatment of Digestive Disease. 1986:425–454. [Google Scholar]
- 15.Shaw BW, Jr, Iwatsuki S, Bron K, et al. Portal vein grafts in hepatic transplantation. Surg Gynecol Obstet. 1985;161:66–68. [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw BW, Jr, Iwatsuki S, Starzl TE. Alternative methods of arterialization of hepatic graft. Surg Gynecol Obstet. 1984;159:490–493. [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw BW, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–534. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw BW, Jr, Starzl TE, Iwatsuki S, et al. An overview of orthotopic transplantation of the liver. In: Flye W, editor. Principles of Organ Transplantation. Philadelphia: W.B. Saunders; 1985. [Google Scholar]
- 19.Starzl TE, Bilheimer DW, Bahnson HT, et al. Heart-liver transplantation in a patient with familial hypercholesterolemia. Lancet. 1984;i:1382–1843. doi: 10.1016/s0140-6736(84)91876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Fung JJ. OKT3 in treatment of allografts rejecting under cyclosporine-steroid therapy. Trans Proc. (submitted) [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Iwatsuki S, Shaw BW., Jr . Transplantation of the human liver. In: Schwarts SI, editor. Abdominal Operations. 8. (Maingot) Appleton-Century-Crofts; Connecticut: 1985. pp. 1687–1722. [Google Scholar]
- 22.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starzl TE, Nalesnik MA, Porter KA, et al. Reversiblity of lymphomas and lymphoproliferative lesions developing under cyclosporine-steroid therapy. Lancet. 1984;i:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzakis AG, Gordon RD, Shaw BW, Jr, et al. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporine era. Transplantation. 1986;40:667–671. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver GZ, Franck WA, Streck WF, et al. Hepatic osteodystrophy after liver transplantation in patients with primary biliary cirrhosis. Am J Gastroenterol. 1983;78:102–106. [PubMed] [Google Scholar]
- 26.Zitelli BJ, Malatack JJ, Garatner JC, et al. Orthotopic liver transplantation in children with hepatic-based metabolic disease. Transplant Proc. 1983;15:1284–1287. [PMC free article] [PubMed] [Google Scholar]


