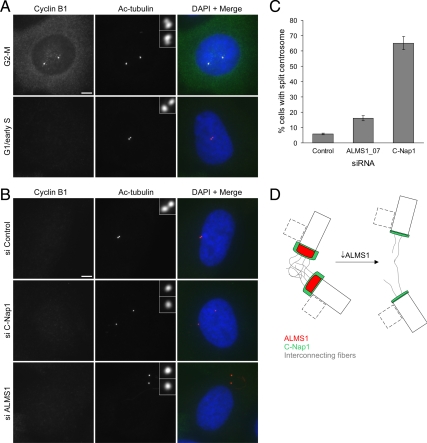
Figure 9.
Depletion of ALMS1 causes centrosome splitting. (A) Cyclin B1 immunostaining was used to determine cell cycle stage, allowing cells entering mitosis to be excluded from analysis. Examples of control cells at the G2-M transition and in G1/early S phase are shown. Centrioles were visualized by costaining with an antibody to acetylated tubulin. Note the separating centrosomes (each containing one parental and one progeny centriole) at G2-M and the close association of the two parental centrioles in G1/early S phase. (B) Immunofluorescence microscopy analysis of centrosome splitting in G1/early S phase cells. Cells were treated with the indicated siRNAs and costained with antibodies to cyclin B1 and acetylated tubulin. Interphase cells with undetectable cyclin B1 and with centrioles >2 μm apart were classed as having a split centrosome. (C) Quantification of centrosome splitting, after siRNA treatment, in interphase cells with undetectable cyclin B1. Depletion of the centrosome cohesion protein C-Nap1 was used as a positive control. The mean ± SE of three independent experiments is shown; in each experiment at least 100 cells were counted for each condition. (D) Model of ALMS1 function in centrosome cohesion, based on the model of C-Nap1/rootletin–dependent centrosome cohesion proposed by Bahe et al. (2005). Depletion of ALMS1 causes, by an unknown mechanism, reductions in the level of C-Nap1 at centrioles. This is predicted to perturb docking of interconnecting or “entangling” fibers with the proximal end of each centriole, leading to centrosome splitting. Procentrioles, which assemble orthogonally to parental centrioles during S and G2 phase, are depicted by dashed lines. Note that C-Nap1 is not thought to localize to the interface between the procentriole and centriole or be involved in maintaining their attachment (Mayor et al., 2000); it is not known if ALMS1 localizes to this interface.