We demonstrate a novel link between APC and hypoxia and show that APC and HIF-1α; antagonize each other. Hypoxia represses APC mRNA via HIF-1α. On the other hand, APC-mediated repression of HIF-1α requires wild-type APC, low levels of β-catenin, and NF-κB activity.
Abstract
The tumor suppressor adenomatous polyposis coli (APC) is mutated in the majority of colorectal cancers and is best known for its role as a scaffold in a Wnt-regulated protein complex that determines the availability of β-catenin. Another common feature of solid tumors is the presence of hypoxia as indicated by the up-regulation of hypoxia-inducible factors (HIFs) such as HIF-1α. Here, we demonstrate a novel link between APC and hypoxia and show that APC and HIF-1α antagonize each other. Hypoxia results in reduced levels of APC mRNA and protein via a HIF-1α–dependent mechanism. HIF-1α represses the APC gene via a functional hypoxia-responsive element on the APC promoter. In contrast, APC-mediated repression of HIF-1α requires wild-type APC, low levels of β-catenin, and nuclear factor-κB activity. These results reveal down-regulation of APC as a new mechanism that contributes to the survival advantage induced by hypoxia and also show that loss of APC mutations produces a survival advantage by mimicking hypoxic conditions.
INTRODUCTION
The adenomatous polyposis coli (APC) tumor suppressor is the most commonly mutated gene in colorectal cancer (Powell et al., 1992). The APC protein is involved in many of the fundamental processes that govern normal gut epithelium. It is best known for controlling the Wnt/β–catenin pathway, where it regulates β-catenin levels, thereby regulating the transcriptional activity of T-cell factor (TCF)/lymphoid-enhancing factor (LEF) transcription factors (Bienz and Clevers, 2000). APC also contributes to the regulation of cytoskeletal proteins (McCartney and Nathke, 2008). Importantly, APC is mutated in the human syndrome familial adenomatous polyposis (FAP). FAP patients are heterozygous for APC and develop hundreds of polyps in their gut that invariably become cancerous if left untreated (Ichii et al., 1993; Fearnhead et al., 2001). The progression to malignancy probably involves inflammation and hypoxia (O'Byrne et al., 2000; Rajaganeshan et al., 2008).
Hypoxia is an important stimulus for tumor angiogenesis and growth (O'Byrne et al., 2000; Garcia, 2006). The transcriptional response to hypoxia is mainly controlled by the hypoxia-inducible factor (HIF) system (Bardos and Ashcroft, 2005). HIF is a heterodimeric transcription factor composed of α and β subunits. Although HIF-1β is constitutively expressed, HIF-α subunits are extremely labile at normal oxygen levels. HIF-1α levels respond to oxygen through posttranslational hydroxylation, which is catalyzed by a class of 2-oxoglutarate dioxygenases called prolyl-hydroxylases (PHDs). Hydroxylation of specific proline residues in the oxygen-dependent degradation domain of HIF-1α targets it for ubiquitination by the Von Hippel Lindau system and subsequent degradation by the proteasome (Fandrey et al., 2006; Bruegge et al., 2007). When oxygen levels are reduced, or cofactors such as iron ions are not available, PHD activity is inhibited so that HIF-1α levels increase, translocates into the nucleus, and transactivates its target genes. Among the HIF-1α targets are PHD2 and PHD3, which create a negative feedback loop for the system (Metzen et al., 2005; Pescador et al., 2005).
The HIF-1α gene is under the control of nuclear factor (NF)-κB (Gorlach and Bonello, 2008; Rius et al., 2008; van Uden et al., 2008) and the chromatin remodelling complex SWI/SNF (Kenneth et al., 2009). NF-κB is the collective name for a family of important transcription factors that control many cellular processes, such as apoptosis and proliferation (reviewed in Perkins and Gilmore, 2006). Impairment of NF-κB results in the loss of HIF-1α mRNA (Rius et al., 2008; van Uden et al., 2008), which reduces HIF-1α levels in response to hypoxia or proteasomal inhibition (Rius et al., 2008; van Uden et al., 2008). This causes inappropriate cellular responses to hypoxia in cells lacking normal NF-κB (van Uden et al., 2008; Kenneth et al., 2009).
HIF-1α and β-catenin are also functionally connected (Kaidi et al., 2007; Lim et al., 2008). Specifically, HIF-1α can interfere with coactivation of TCF/LEF transcription mediated by β-catenin. Furthermore, β-catenin can bind and regulate NF-κB activity (Deng et al., 2002, 2004; Du et al., 2009). Despite the established relationship between HIF-1α, NF-κB, and Wnt/β-catenin, a link between APC and HIF-1α has not been investigated.
Here, we report functional cross-talk between HIF-1α and APC at the transcriptional level. Depletion of HIF-1α results in increased APC mRNA and protein. Consistent with direct transcriptional repression of APC by HIF-1α, we discovered a hypoxia-responsive element (HRE) in the APC promoter and demonstrate that hypoxia induces HIF-1α binding to this site. Importantly, hypoxia promotes a reduction in APC mRNA and protein in different cells suggesting that suppression of APC by hypoxia may be involved in increased survival in hypoxic conditions in tumors with wild-type APC. Interestingly, APC depletion results in increased HIF-1α levels and activity. This increase is mediated by NF-κB and requires regulation of β-catenin by APC. Our results also suggest that cells lacking APC are adapted to hypoxia and hence have a survival and proliferative advantage under hypoxic conditions. This could be an important factor in the progression of colorectal tumors.
MATERIALS AND METHODS
Cells
U2OS were obtained from American Type Culture Collection (Manassas, VA). HCT-116 (parental, HAβ 85, and HAβ 18) were a kind gift from Prof. T. Waldman (Georgetown University, Washington DC). SW480 and DLD-1 (Li and Nathke, 2005) were grown in DMEM (Lonza Verviers, Verviers, Belgium) supplemented with 10% fetal bovine serum (Invitrogen, Paisley, United Kingdom), 50 U/ml penicillin (Lonza Verviers), and 50 μg/ml streptomycin (Lonza Verviers) for no >30 passages. U2OS-HRE luciferase cells were a kind gift from Dr. Margaret Ashcroft (University College, London, UK) and have been described previously (Bardos et al., 2004; Kenneth et al., 2009).
Small Interfering RNA (siRNA) Transfection
siRNA duplex oligonucleotides were synthesized by Eurofins MWG Operon (Huntsville, AL) and transfected using Oligofectamine (Invitrogen) and INTERFERin (Polyplus, Illkirch, France) as per the manufacturer's instructions. siRNA sequences are as follows: control, AACAGUCGCGUUUGCGACUGG (van Uden et al., 2008); (a) HIF-1α-CUGAUGACCAGCAACUUGA (van Uden et al., 2008) and (b) HIF-1α-GGAAUUGGAACAUUAUUAC; APC (Dharmacon RNA Technologies, Lafayette, CO) (Dikovskaya et al., 2007); and RelA, GCUGAUGUGCACCGACAAG (Anderson and Perkins, 2003). Unless stated, HIF-1α sequence (a) was mostly used throughout this study.
Mouse Tissue and Staining
Tissue samples of mouse small intestine were a kind gift from Dr. Owen Sansom (CRUK Beatson Institute, Glasgow, United Kingdom). Tissue was harvested from (AhCre+ Apcfl/fl) mice 4 d after APC deletion from the intestinal crypt and stem cells (Sansom et al., 2004). APC was deleted from the intestinal epithelium by inducing Cre-mediated recombination with β-naphtoflavone as described previously (Sansom et al., 2004), and mice were treated with 20 mg/kg i.p. Taxol (paclitaxel, Mayne Pharma, Salisbury South, SA. Australia), a microtubule stabilizer 3 h (h) before tissue was harvested. Taxol treatment arrests cell in mitosis and makes APC-depleted regions of the gut more visible. Gut tissue was fixed in 4% paraformaldehyde and processed into wax blocks. Dewaxed sections were placed in citrate buffer and antigen retrieved in a pressure cooker. After cooling overnight, slides were rinsed in phosphate-buffered saline (PBS) 2 × 10 min and permeabilized with 1% Triton X-100 in PBS for 20 min. After a rinse in PBS, slides were blocked for 2 h with MAXblock (Active Motif, Carlsbad, CA) at room temperature. Slides were incubated with CA9 antibody at a dilution of 1:500 in working buffer (WB: 0.1% bovine serum albumin, 0.3% normal goat serum, and 0.2% Triton X-100 in PBS, pH 7.4) overnight at 4°C. After 5 × 5-min rinses in WB, slides were incubated in secondary antibody goat ant-rabbit Alexa 647 (Invitrogen; 1:250) for 1 h at room temperature. After 5 × 5-min rinses in WB and 3 × 5-min rinses in PBS, cell nuclei were counterstained with 4,6-diamidino-2-phenylindole in PBS. After rinsing in PBS, sections were mounted in Prolong Gold (Invitrogen). Slides were imaged on a DeltaVision Core microscope system (Applied Precision, Seattle, WA), and images were deconvolved using softWoRx (Applied Precision).
Hypoxia Inductions and MG132 Treatment
Cells were incubated at 1% O2 in an InVIVO 300 hypoxia workstation (Ruskin Technologies, Bridgend, United Kingdom). Cells were lysed for protein and RNA extraction in the workstation to avoid reoxygenation. MG132 (Merck Biosciences, Darmstadt, Germany) at 50 μM was added 3 h before cell harvesting.
Chromatin Immunoprecipitation (ChIP)
Proteins were cross-linked with formaldehyde for 10 min. Then, 0.125 mol/l glycine was added, and cells were washed with PBS. Cells were lysed with lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1, 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml leupeptin, and 1 mg/ml aprotonin), followed by sonication and centrifugation. The supernatant was precleared with sheared salmon sperm DNA and protein G-Sepharose beads (Sigma Chemical/Generon, Berkshire, UK). Supernatant was incubated with specific antibodies overnight and then with protein G-Sepharose beads for 1 h. After extensive wash step, the complexes were eluted with buffer (100 mmol/l NaHCO3 and 1% SDS) and incubated with proteinase K. DNA was purified using polymerase chain reaction (PCR) purification kit (QIAGEN/National Blood Service, Burmingham, United Kingdom). PCR was performed using the following primers: CA9 promoter (HRE), forward, GACAAACCTGTGAGACTTTGGCTCC and reverse, AGTGACAGCAGCAGTTGCACAGTG; and APC HRE2, forward, TAGGGCTAGGCAGGCTGTG and reverse, CTGCACCAATACAGCCACAT.
Colony Formation Assay
Cells were plated at 5000 or 10,000 cells per well of six-well plates and grown for 8 d after which cells were washed with PBS and stained with crystal violet. Plates were scanned, and number of colonies was counted using ImageJ software (National Institutes of Health, Bethesda, MD).
Antibodies
Antibodies used were as follows: HIF-1α (MAB1536, R&D Systems), CA9 (NB100-417; Novus Biologicals. Littleton, CO), β-actin (A5441; Sigma Chemical), Glut3 (RB-9096; Neomarkers, Fremont, CA), RelA (sc-372; Santa Cruz Biotechnology, Santa Cruz, CA), phospho-Ser536 RelA (3033; Cell Signaling Technology, Danvers, MA), phospho-Ser32/36 inhibitor of κB (IκB-α) (9246; Cell Signaling Technology), IκB-α (4812; Cell Signaling Technology), HIF-1β (3718; Cell Signaling Technology), APC (Nathke et al., 1996), α-tubulin (T9026; Sigma Chemical), β-catenin (4270; Cell Signaling Technology; Dikovskaya et al., 2007), BNIP3 (Ab10433; Abcam, Cambridge, United Kingdom), Chk1 (sc-8408; Santa Cruz Biotechnology), cyclin D1 (2926; Cell Signaling Technology), and LC3B (2775; Cell Signaling Technology or sc-16756; Santa Cruz Biotechnology). ChIP antibodies used were as follows: HIF-1α (sc-10790; Santa Cruz Biotechnology), HIF-1β (sc-5580; Santa Cruz Biotechnology), acetyl-H3 (06-599; Millipore, Billerica, MA), and polymerase II (Pol II) carboxy-terminal domain (sc-47701; Santa Cruz Biotechnology).
RNA, cDNA, and Quantitative (q)PCR
Total RNA was extracted using Invisorb spin cell RNA (Invitek, Hayward, CA) or PeqLab Gold RNA extraction kit, according to the manufacturer's directions. RNA was converted to cDNA using Quantitect reverse transcription kit (QIAGEN). Brilliant II SYBR Green kit (Stratagene/Agilent Technologies, Santa Clara, CA) was used, and samples analyzed using a Mx3005P qPCR machine and software (Stratagene/Agilent).
qPCR Oligonucleotides Sequences
qPCR oligonucleotides sequences were as follows: actin, forward, CTGGGAGTGGGTGGAGGC and reverse, TCAACTGGTCTCAAGTCAGTG; RelA, forward, CTGCCGGGATGGCTTCTAT and reverse, CCGCTTCTTCACACACTGGAT; HIF-1α, forward, CATAAAGTCTGCAACATGGAAGGT and reverse, ATTTGATGGGTGAGGAATGGGTT; APC, forward, TGTCCCTCCGTTCTTATGGAA and reverse, TCTTGGAAATGAACCCATAGGAA; CA9, forward, CTTTGCCAGAGTTGACGAGG and reverse, CAGCAACTGCTCATAGGCAC; Glut3, forward, CAATGCTCCTGAGAAGATCATAA and reverse, AAAGCGGTTGACGAAGAGT; and ADM, forward, GGAAGAGGGAACTGCGGATGT and reverse, GGCATCCGGACTGCTGTCT.
Statistical Analysis
Analysis of variance and Student's t tests were performed on the means, and p values were calculated (*p ≤ 0.050, **p ≤ 0.010, and ***p ≤ 0.005.
Other Experimental Procedures
Immunoblots, transfections, and luciferase assays have been described previously (Rocha et al., 2005; Schumm et al., 2006; Dikovskaya et al., 2007, and references therein).
RESULTS
APC Is Repressed Directly by HIF-1α during Hypoxia
Hypoxia is a common feature of most solid tumors and is thought to contribute to tumor progression and therapy evasion (Garcia, 2006). In colorectal cancer, APC mutation occurs early and subsequent genetic alterations drive tumorigenesis. Given the importance of hypoxia in cancer, we investigated whether hypoxia modulated APC function in cells. Immunoblotting protein from a variety of cell lines after hypoxia treatment showed that hypoxia resulted in reduced APC protein in all the cells tested regardless of APC mutation status (Figure 1A). In addition, in U2OS cells, which express wild-type APC, hypoxia resulted in increased β-catenin stabilization (Figure 1A).
Figure 1.
HIF-1α represses APC gene expression. (A) U2OS, HCT-116. Haβ18, Haβ85, SW480, and DLD-1 cells were exposed to 1% O2 for 24 h before harvest. Whole cell lysates were analyzed by immunoblot blot using the indicated antibodies. (B) U2OS cells were exposed to 1% O2 for the indicated periods before total RNA or whole cell lysates extraction. RT-qPCR for APC was performed with actin as a reference. The graph depicts the mean plus SD of a minimum of three independent experiments performed in duplicate. Whole cell lysates were analyzed by immunoblot blot using the indicated antibodies. (C) U20S, HCT-116, and HAβ18 cells were transfected with control and HIF-1α siRNA oligonucleotides (a and b) for 48 h before total RNA extraction. RT-qPCR for APC and HIF-1α was performed with actin as a reference gene. The graph depicts the mean plus SD of a minimum of three independent experiments performed in duplicate. (D) U2OS cells were transfected with control, APC, and HIF-1α siRNA oligonucleotides for 48 h. Cells were exposed to 1% O2 for 24 h before harvest. Whole cell lysates were analyzed by Western blot using the indicated antibodies. Asterisk (*) indicates the level of significance. *p < 0.05, **p < 0.01, and ***p < 0.005.
To determine whether hypoxia changed APC gene transcription, we used reverse transcriptase (RT) followed by qPCR to measure APC mRNA at different times after hypoxia exposure (Figure 1B). Our analysis revealed that hypoxia results in decreased APC mRNA levels in a time-dependent manner corresponding to HIF-1α stabilization (Figure 1B and Supplemental Figure 1A).
A key response to hypoxia is activation of the transcription factor HIF-1α. To determine whether hypoxia-mediated APC inhibition was HIF-1α dependent, we depleted HIF-1α by using siRNA and measured APC mRNA levels (Figure 1C). HIF-1α depletion in all cells tested using two different siRNA oligonucleotides, resulted in increased levels of APC mRNA in normoxia (Figure 1C). Similar results were also observed in hypoxia (Supplemental Figure 1B). This correlated with increased APC protein in cells depleted of HIF-1α (Figure 1D). Together, these results indicate that HIF-1α represses APC gene expression.
APC Is a Direct HIF-1α Target
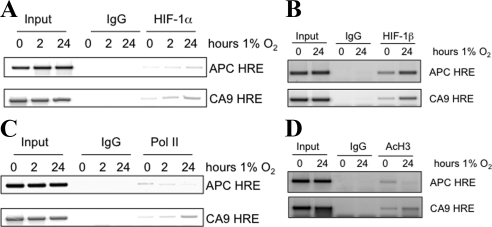
Hypoxia results in reduced expression of APC, and HIF-1α depletion by RNA interference increased APC mRNA and protein levels. To determine whether APC is a direct target of HIF-1α, we analyzed the APC promoter region using MattInspector, which revealed two putative HIF-1α binding sites located in the APC promoter (accession U02509). ChIP using a HIF-1α antibody revealed specific and inducible HIF-1α binding to region 2 but not the putative HIF-1α binding site 1 (Figure 2A; data not shown). The CA9 promoter was used as a positive control. Hypoxia induces rises in HIF-1α protein due to increased stability. As such, it was possible that the observed HIF-1α recruitment to these promoters was merely a consequence of increased protein levels in the cell. To rule out this possibility, we performed ChIP experiments with the HIF-1α binding partner HIF-1β, whose levels are unaltered by hypoxia exposure (Figure 2B). The results clearly demonstrate that hypoxia induces an increased recruitment of HIF-1β to both the CA9 and the APC promoters (Figure 2B), further confirming a direct role for the HIF-1 transcription factor in the repression of the APC promoter. Importantly, binding of HIF-1α to the APC promoter was accompanied by hallmarks of repressed transcription, specifically a reduction in the recruitment of RNA Pol II (Figure 2C) and in the levels of AcH3 after exposure to hypoxia (Figure 2D and Supplemental Figure 2). These experiments revealed that hypoxia-activated HIF-1α represses the APC promoter directly by occupying a specific site on the APC promoter revealing APC as a novel HIF-1α target and providing crucial insight into the poorly understood transcriptional control of this important tumor suppressor.
Figure 2.
APC is a direct HIF-1α target. (A) ChIP analysis for HIF-1α binding to the APC and CA9 promoters. U2OS cells were exposed to 1% O2 for the indicated periods before fixation and lysis. HIF-1α-bound DNA was amplified with specific primers spanning the annotated HRE region on the CA9 and the putative HRE at the APC promoter. Rabbit immunoglobulin G (IgG) was used as a control for the immunoprecipitation. Input represents 10% of the starting material used per immunoprecipitation (IP). (B) ChIP analysis for HIF-1β binding at the APC and CA9 promoters. U2OS cells were exposed to 1% O2 for 24 h before fixation and lysis. HIF-1β–bound DNA was amplified with specific primers spanning the annotated HRE region on the CA9 and the putative HRE at the APC promoter. Rabbit IgG was used as a control for the immunoprecipitation. Input represents 10% of the starting material used per IP. (C) ChIP for AcH3. (D) Polymerase II at the APC HRE site. Cells were treated and processed as described in B. Rabbit IgG was used as a negative control. APC HRE site was amplified using specific PCR primers. Inputs represent 10% of starting material used in each IP.
APC Depletion Increases HIF-1α Message, Protein, and Transcriptional Activity
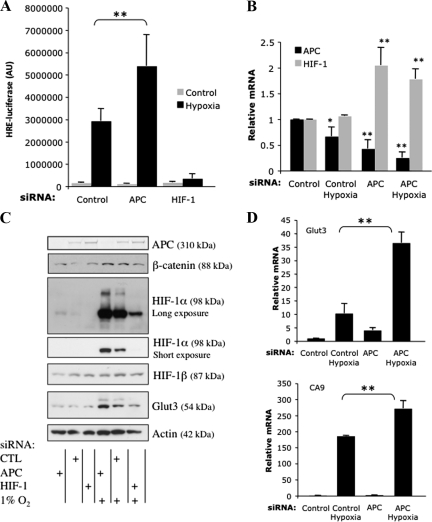
To determine the effect of the APC tumor suppressor on the activity of HIF-1α, we performed luciferase reporter assays in a U2OS reporter cell line that has been used extensively to measure HIF-1α transcriptional activity (Bardos et al., 2004; Kenneth et al., 2009). Cells were transfected with previously validated siRNA oligonucleotides that target APC (Dikovskaya et al., 2007) and HIF-1α (Kenneth et al., 2009). In addition, cells were exposed to 1% O2 for 24 h before harvest. As expected, depletion of HIF-1α abolished hypoxia-induced luciferase activity, confirming loss of HIF-1α (Figure 3A). Depletion of APC, in contrast, resulted in a significant increase in HIF-1α activity (Figure 3A).
Figure 3.
APC depletion increases HIF-1 levels and activity. (A) U2OS-HRE luciferase cells were transfected with the indicated siRNA oligonucleotides. Where indicated, cells were exposed to 1% O2 for 24 h before harvest. Luciferase activity was analyzed 48 h posttransfection. Graph depicts the mean plus SD of a minimum of two independent experiments performed in duplicate. (B) U2OS cells were transfected with control and APC siRNA oligonucleotides for 48 h before total RNA extraction. Where indicated, cells were exposed to 1% O2 for 24 h. After cDNA synthesis, qPCR was performed for APC and HIF-1α mRNA. Levels were normalized to actin mRNA, and the graph depicts the mean plus SD of a minimum of three independent experiments performed in duplicate. (C) U2OS cells were transfected with siRNA oligonucleotides as indicated and exposed or not to 1% O2 for 24 h before harvest. Whole cell lysates were analyzed by Western blot to detect the levels of APC, HIF-1α, and the HIF-1α target Glut3. Actin and HIF-1β were used as loading controls. (D) U2OS cells were treated as described in B, and qPCR was performed for the HIF-1α target genes as indicated. Asterisk (*) indicates the level of significance. *p < 0.05, **p < 0.01, and ***p < 0.005.
To establish whether the mechanism responsible for increased HIF-1α transcriptional activity in response to APC loss involved transcriptional changes, APC levels were depleted using siRNA oligonucleotides and total RNA was extracted. RT-qPCR was used to measure APC and HIF-1α mRNA levels. APC siRNA reduced APC mRNA significantly in U2OS cells (Figure 3B). Surprisingly, this reduction in APC was accompanied by a significant increase of HIF-1α mRNA (Figure 3B). These effects also were observed after hypoxia treatment (Figure 3B). Slight changes in HIF-1α mRNA can cause significant changes in HIF-1α protein levels and activity (van Uden et al., 2008; Kenneth et al., 2009), so we determined whether APC depletion also increased HIF-1α protein and its endogenous target genes. U2OS cells were transfected with APC siRNA and exposed to 1% O2 for 24 h before harvest. Immunoblotting showed that APC depletion produced an increase in basal levels of HIF-1α and a further increase in hypoxia-stabilized HIF-1α protein (Figure 3C). Similar results were observed when cells were treated with the proteasome inhibitor MG132, where APC depletion resulted in higher HIF-1α protein levels (Supplemental Figure 3A). In addition, the Glut3 protein (a known HIF-1α target) was also increased when APC was absent (Figure 3C). Importantly, transcripts of several HIF-1α target genes such as Glut3, CA9, PHD2, and ADM also were increased both in normoxia and hypoxia when APC was depleted (Figure 3D and Supplemental Figure 3B). These results establish that APC depletion leads to HIF-1α activation.
APC Modulation of HIF-1α Requires Wild-Type APC
To determine whether our findings were also evident at the organism level, we investigated the effects of APC depletion in vivo. Using tissue harvested from AhCre+ Apcfl/fl mice 4 d after APC deletion from the intestinal crypt, we visualized the levels of the HIF-1α target CA9 (Sansom et al., 2004). In wild-type tissue, CA9 was localized to the cell cortex in differentiated cells and only weakly detectable in crypts (Figure 4A). APC depletion in cells toward the base of the crypt resulted in disorganized epithelium (Sansom et al., 2004) with increased CA9 staining indicating higher HIF-1α activity (Figure 4A). Because Cre-activation and thus APC depletion is initiated in the proliferative compartment of the crypt, cells toward the tip of the villus in these sections are still wild type for APC (Sansom et al., 2004) and, like control tissue, they showed only weak staining for CA9. These results indicate that APC depletion results in activation of HIF-1α in the context of a tissue confirming our biochemical data.
Figure 4.
Depletion of APC increases HIF-1α activity in situ. (A) CA9 staining of wild-type (WT) and APC-depleted mouse small intestine. Maximum intensity projections of widefield, deconvolved images show cortical localization of CA9 in crypts from WT tissue (arrowhead), except in Paneth cells at the base of the crypt, which show little or no staining. APC-depleted cells toward the base of the crypt/villus axis (arrow) form a disorganized epithelium and elevated CA9 homogenously in the cytoplasm. Because Cre-activation and thus APC depletion only occurs in the proliferative compartment of the crypt, the same tissue section also shows cells toward the tip of the villus (arrowhead) that are wild type for APC, and, like control tissue, show only weak staining for CA9. Bars, 20 μm. (B) SW480 and DLD-1 cells were transfected with control and APC siRNA oligonucleotides and treated where indicated with 50 μM MG132 for 3 h before harvest. Whole cell lysates were analyzed by Western blot using the indicated antibodies.
APC is mutated in the majority of colorectal cancers and these tumors often have elevated HIF-1α protein levels (Furlan et al., 2008). To establish whether mutant APC can still regulate HIF-1α, we determined whether depletion of mutant APC also resulted in increased HIF-1α levels. For this purpose, we analyzed SW480 and DLD-1 cells. Both express only truncated, mutant APC (Li and Nathke, 2005; Figure 4B). Because HIF-1α protein is usually kept at low levels by O2-dependent proteasomal degradation, any changes in HIF-1α mRNA can be visualized using the proteasome inhibitor MG132 (Bardos et al., 2004; Kenneth et al., 2009). When APC was depleted in either of these cell types, no changes in HIF-1α protein were detected (Figure 4B), suggesting that mutant APC cannot modulate HIF-1α levels.
APC Modulation of HIF-1α Requires Wild-Type β-Catenin
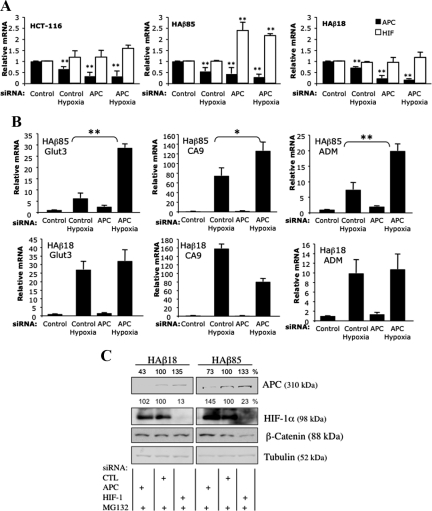
One of the main functions of APC is to limit Wnt/β–catenin signaling, so it was important to determine whether increases in HIF-1α induced by depletion of APC were due to changes in β-catenin. Using a panel of isogenic HCT-116 cells that express either mutant (stabilized and thus not sensitive to APC regulation) and wild type β-catenin (parental), or only wild-type β-catenin (HAβ85), or only mutant (HAβ18) β-catenin, we repeated the depletion of APC, treated with hypoxia, and measured HIF-1α levels under these conditions. RT-qPCR analysis revealed that although in parental and mutant only β-catenin (HAβ18) cells, APC depletion did not cause a change in HIF-1α mRNA, depletion of APC in wild-type only β-catenin (HAβ85) cells resulted in increased HIF-1α mRNA (Figure 5A). In addition, we investigated whether increased HIF-1α mRNA translated to increased HIF-1α activity (Figure 5B). In cells containing wild-type β-catenin, depletion of APC resulted in significant increases in HIF-1α target genes such as Glut3, CA9, ADM, PHD2, and BNIP3 (Figure 5B and Supplemental Figure 4). However, in the presence of mutant β-catenin, depletion of APC did not cause any significant changes in HIF-1α target genes (Figure 5B and Supplemental Figure 4). Additional analysis revealed that APC depletion in β-catenin wild-type cells resulted in more stabilized HIF-1α after MG132 treatment (Figure 5C). Together, these results indicate that APC modulation of HIF-1α mRNA requires intact regulation of β-catenin by APC.
Figure 5.
Increased HIF-1α after APC depletion requires wild-type β-catenin. (A) HCT-116 (parental, mutant, and wild-type β-catenin), HAβ85 (wild-type β-catenin), and HAβ18 (mutant β-catenin) were transfected with control or APC siRNA oligonucleotides for 48 h before RNA extraction and exposed or not to 1% O2 for 24 h. RT-qPCR was performed for APC and HIF-1α, with actin used as a normalizing gene. The graph depicts mean plus SD of a minimum of three independent experiments performed in duplicate. (B) HAβ85 (wild-type β-catenin) and HAβ18 (mutant β-catenin) were transfected with control or APC siRNA oligonucleotides for 48 h before RNA extraction and exposed or not to 1% O2 for 24 h. RT-qPCR was performed for the indicated HIF-1α target genes. The graph depicts mean plus SD of a minimum of three independent experiments performed in duplicate. Asterisk (*) indicates the level of significance. *p < 0.05, **p < 0.01, and ***p < 0.005. (C) HAβ85 (wild-type β-catenin) and HAβ18 (mutant β-catenin) were transfected with control, APC, or HIF-1α siRNA oligonucleotides and treated where indicated with 50 μM MG132 for 3 h before harvest. Whole cell lysates were analyzed by immunoblot using the indicated antibodies. ImageJ software was used to quantify band intensity.
APC/β-Catenin Modulation of HIF-1α Depends on NF-κB
We showed previously that the HIF-1α gene is under the tight control of NF-κB (van Uden et al., 2008; Kenneth et al., 2009). NF-κB is usually retained in the cytoplasm by the action of a family of proteins called IκBs. On activating stimuli such as tumor necrosis factor (TNF)-α, IκB is degraded so NF-κB can translocate into the nucleus and active its targets (Perkins and Gilmore, 2006). The NF-κB response in the isogenic cell lines we used to investigate the role of β-catenin in modulating of HIF-1α was described recently (Du et al., 2009). The Geller group demonstrated that Wnt/β-catenin regulates cytokine-induced NF-κB activation (Du et al., 2009). We analyzed NF-κB activation in response to TNF-α in these cells by using a biochemical fractionation assay and immunofluorescence, to measure RelA translocation from the cytoplasm into the nucleus (Supplemental Figure 5, A and B). We confirmed that in wild-type β-catenin cells, TNF-α induced p65/RelA nuclear translocation, whereas in the parental HCT-116 cells and mutant β-catenin only cells, RelA nuclear translocation was attenuated (Supplemental Figure 5, A and B). In addition, the level of phosphorylated RelA at Ser 536, a marker of NF-κB activity, was also higher in wild-type β-catenin cells. Interestingly, phosphorylation of the NF-κB inhibitory protein IκB-α also was diminished in β-catenin mutant cells (Supplemental Figure 5C).
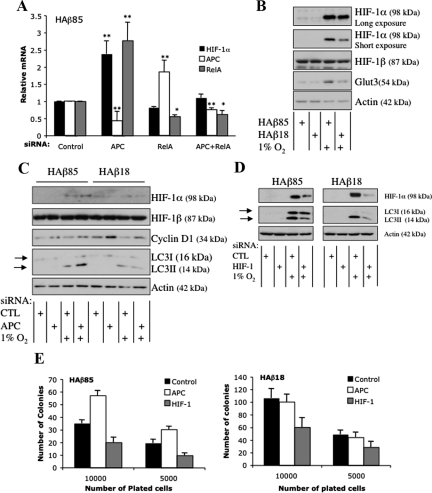
We next investigated whether in cell lines expressing wild-type β-catenin, NF-κB is involved in the APC-mediated modulation of HIF-1α. We depleted RelA in combination with APC by using siRNA. In HAβ85 cells, siRNA targeted against RelA and APC efficiently reduced RelA and APC protein by ∼50% (Figure 6A). Importantly, codepletion of APC and RelA completely prevented the increase in HIF-1α mRNA achieved by APC depletion alone. Similar results were also obtained in U2OS cells (Supplemental Figure 6). Together, our data show that elevated wild-type β-catenin and the subsequent increase in NF-κB that result from APC depletion up-regulate the HIF-1α gene.
Figure 6.
Increased HIF-1α after APC depletion requires NF-κB. (A) HAβ85 cells were transfected with the indicated siRNA oligonucleotides for 48 h before RNA extraction. Where indicated, cells were exposed to 1% O2 for 24 h. RT-qPCR was performed for the levels of HIF-1α, RelA, and APC, with actin used as normalizing gene. Graph depicts the mean plus SD of a minimum of three independent experiments performed in duplicate. (B) HAβ85 and HAβ18 cells were treated with 1% O2 for 24 h before lysis. Whole cell lysates were analyzed by immunoblot for the levels of the indicated proteins. (C) HAβ85 and HAβ18 cells transfected with control and APC siRNA oligonucleotides for 48 h. Cells were exposed to 1% O2 for 24 h before harvest. Whole cell lysates were analyzed by Western blot using the indicated antibodies. Arrows indicate LC3I and LC3II. (D) HAβ85 and HAβ18 cells transfected with control and HIF-1α siRNA oligonucleotides for 48 h. Cells were exposed to 1% O2 for 24 h before harvest. Whole cell lysates were analyzed by Western blot using the indicated antibodies. Arrows indicate LC3I and LC3II. (E) Survival effects of APC and HIF-1α depletion in HAβ85 and HAβ18 cells. Cells were transfected with siRNA oligonucleotides for APC and HIF-1α as indicated. 24 h posttransfection, cells were counted and the indicated number of cells were plated and allowed to grow for additional 7 d before fixation and crystal violet staining. Plates were scanned and ImageJ software was used to count colonies.
APC Depletion Enhances HIF-1α–mediated Cellular Functions
We confirmed the effect of deregulated β-catenin on HIF-1α protein by using the isogenic HCT-116 cell lines. Consistent with our mRNA data, we found cells with wild-type β-catenin have higher levels of HIF-1α compared with those with mutant β-catenin cells (Figure 6B). This was mirrored by HIF-1α activity as indicated by elevated levels of Glut3 in wild-type cells (Figure 6B).
Hypoxia induces several cellular changes, including autophagy (Kenneth and Rocha, 2008). Autophagy is characterized by the formation of autophagosomes, which recycle damaged organelles such as mitochondria, and it can contribute to both cell survival and death (Levine and Kroemer, 2008; Yu et al., 2008). During autophagy, light chain 3 (LC3) proteins are cleaved from LC3I and LC3II forms, and the cleaved form associates with autophagosomes. The presence of LC3II on autophagosomes as well as its conversion to its cleaved form are indicators of autophagy (Morselli et al., 2009).
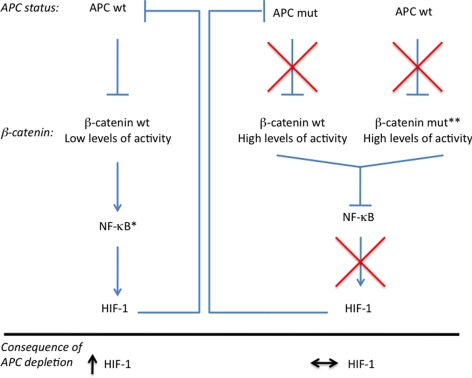
Because our results suggested that APC depletion enhances HIF-1α levels, we next investigated whether this correlated with changes in levels of the autophagy marker LC3II. When APC was depleted in wild-type β-catenin cells that were exposed to hypoxic conditions, LC3II levels were higher than in control cells (Figure 6C). However, in β-catenin mutant cells, depletion of APC failed to increase this autophagy marker (Figure 6C). Nevertheless, mutant β-catenin cells when depleted of APC, still up-regulate Wnt target genes such as cyclin D1 (Figure 6C). To determine the contribution of HIF-1α to hypoxia-induced autophagy, we depleted cells of HIF-1α before hypoxia exposure. In the absence of HIF-1α, hypoxia-induced cleavage of LC3 was greatly reduced (Figure 6D). This is in accordance with previous studies where HIF-1α has been shown to play a prominent role in hypoxia-mediated autophagy (Zhang et al., 2008; Wilkinson and Ryan, 2009; Liu et al., 2010; Menrad et al., 2010). The effects of APC and HIF-1α in the two different cell lines also were confirmed using LC3 immunofluorescence (Figure S7). These results suggest that APC depletion enhances HIF-1α–mediated autophagy in cells with an intact APC–β-catenin regulatory pathway. In addition, we verified that APC and HIF-1α depletion resulted in increased survival, proliferation, or both in these cells (Figure 6E). We found that APC depletion resulted in increased colony numbers only in cells with wild-type β-catenin. In contrast, HIF-1α depletion resulted in reduced colony formation in both cell types, even though the effect was more prominent in cells with wild-type β-catenin (Figure 6E). These results demonstrate that the level of APC and HIF-1α directly affects the survival and proliferation of cells (Figure 7).
Figure 7.
Summary diagram of key findings. HIF-1α represses the APC gene directly. APC-mediated repression of HIF-1α is indirect and requires wild-type APC, low levels of β-catenin, and NF-κB activity. *, low levels of β-catenin allow for NF-κB activity and hence are not repressed. **, mutations in β-catenin make this protein nondegradable and thus constitutively active. High levels of β-catenin prevent NF-κB activation.
DISCUSSION
In this report, we uncovered an antagonistic relationship between the tumor suppressor APC and the hypoxia activated transcription factor HIF-1α, two important genes involved in development as well as in cancer progression and suppression. Although HIF-1α represses the APC gene directly, APC-mediated repression of HIF-1α relies on controlled changes in the levels of β-catenin and NF-κB.
We found that HIF-1α represses the APC gene directly via a functional HRE in the APC promoter (Figures 1 and 2 and Supplemental Figures 1 and 2). In this case, HIF-1α binding results in repression of APC mRNA and protein. This reveals an important mechanism for how hypoxia can promote cell survival. HIF-1α–repressive activities are not well understood. However, its ability to repress DNA repair genes such as MSH2 and MSH6 is established (Yoo et al., 2009). Our data thus uncovered APC as a novel HIF-1α–repressed gene. We observed that hypoxia induced the repression of wild-type and mutant APC. The consequence of losing mutated APC in hypoxic tumor cells is not clear. It is possible that mutant APC has functions that are different from wild-type APC. However, such pathways have not been fully elucidated, but they may involve other aspects of APC biology such as cytoskeletal regulation. In the future, it will be necessary to investigate how non-Wnt–related processes mediated by APC are affected by inhibition of mutant APC after hypoxia.
A crucial function of APC in cells is to limit the amount of β-catenin available for transcriptional activation and this is induced by Wnt signaling. However, recent studies have shown that after inactivation of APC, nuclear accumulation of β-catenin occurs later and at more advanced stages of disease (Phelps et al., 2009). Furthermore, β-catenin is not uniformly nuclear in cells lacking APC and also can be affected by other factors, including the extracellular matrix and p53 (Brabletz et al., 2001; Damalas et al., 2001; Liu et al., 2001). This suggests that cytoplasmic β-catenin also may play a role in other signaling pathways that can contribute to cancer progression. Consistent with this idea, recent studies showed that β-catenin can alter NF-κB function (Deng et al., 2002, 2004; Du et al., 2009): whereas high levels of β-catenin (found in mutant APC or mutant β-catenin–expressing cells) inhibit NF-κB activity, cells with low levels of β-catenin have normal NF-κB activity (Du et al., 2009). We were able to reproduce these results using HCT-116 cell lines that express either wild-type or mutated (stabilized) β-catenin (Supplemental Figure 5). Our data indicate that depletion of APC in cells with wild-type β-catenin leads to NF-κB–mediated activation of the HIF-1α gene. Although paradoxical (because the elevated levels of β-catenin that result from inactivation of APC are expected to inhibit NF-κB), this observation might explain why inhibition of APC alone does not immediately give rise to malignant tumors. In this scenario, the elevated levels of HIF-1α could exert a protective effect against tumorigenesis as observed in mouse models of chemical induced carcinogenesis (Taylor and Colgan, 2007). Furthermore, HIF-1α can control β-catenin function (Kaidi et al., 2007) and inhibit a variety of TCF4 target genes, including c-myc and cyclin D1 (Lim et al., 2008). This means that even after inhibition of APC, the resulting upregulation of HIF-1α could delay Wnt signaling effects. However, the situation can be reversed after prolonged lack of APC or in the presence of constitutively high levels of β-catenin. In fact, in cells acclimatized to APC inhibition or with high levels of β-catenin, HIF-1α promotes Xenograft growth (Dang et al., 2006). In this context, it is noteworthy that although cells that express only mutant β-catenin do not up-regulate HIF-1α to the same extent as wild-type β-catenin–expressing cells when depleted of APC (Figure 6B), they still up-regulate Wnt target genes such as cyclin D1 (Figure 6C) and they respond with a measurable, albeit smaller, increase in β-catenin transcriptional activity. This may be due to a loss of APC from the cytoplasm where it could normally act to sequester β-catenin. Significantly, we show that in the context of a tissue, depletion of APC results in increased HIF-1α activity (Figure 4A), thus establishing a setting for our findings at an organism level.
The dual role of NF-κB as either a tumor suppressor or an oncogene has been extensively described previously (Perkins and Gilmore, 2006). We found that depleting APC leads to NF-κB–mediated activation of HIF-1α, whereas other studies showed that APC overexpression can activate NF-κB (Deng et al., 2004). A more detailed analysis of the relative activity of these different molecules at different stages of tumor progression is needed to determine how the balance of APC, β-catenin, and NF-κB contributes to tumorigenesis. Importantly, our results suggest that prolonged hypoxia can induce oncogenic changes in tumors that retain wild-type APC and β-catenin.
Supplementary Material
ACKNOWLEDGMENTS
We thank Prof. T. Waldman for providing the HAβ cells and Dr. O. Sansom for providing the APC-Cre mouse tissue. This study was mainly funded by a Cancer Research UK program grant (to I.P.N., P.L.A., and I.S.N.) and Association for International Cancer Research project grant (to N.S.K.). S. R. is funded by a Research Council UK fellowship, University of Dundee, with additional support from a Medical Research Council New Investigator Research Grant.
Abbreviations used:
- APC
adenomatous polyposis coli
- ChIP
chromatin immunoprecipitation
- HIF
hypoxia-inducible factor
- HRE
hypoxia-responsive element
- LC3
light chain 3
- PHD
prolyl-hydroxylase
- Pol II
polymerase II
- qPCR
quantitative polymerase chain reaction
- RT
reverse transcriptase.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-04-0312) on September 15, 2010.
REFERENCES
- Anderson L. A., Perkins N. D. Regulation of RelA (p65) function by the large subunit of replication factor C. Mol. Cell Biol. 2003;23:721–732. doi: 10.1128/MCB.23.2.721-732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardos J. I., Ashcroft M. Negative and positive regulation of HIF-1, a complex network. Biochim. Biophys. Acta. 2005;1755:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bardos J. I., Chau N. M., Ashcroft M. Growth factor-mediated induction of HDM2 positively regulates hypoxia-inducible factor 1alpha expression. Mol. Cell Biol. 2004;24:2905–2914. doi: 10.1128/MCB.24.7.2905-2914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Brabletz T., Jung A., Reu S., Porzner M., Hlubek F., Kunz-Schughart L. A., Knuechel R., Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruegge K., Jelkmann W., Metzen E. Hydroxylation of hypoxia-inducible transcription factors and chemical compounds targeting the HIF-alpha hydroxylases. Curr. Med. Chem. 2007;14:1853–1862. doi: 10.2174/092986707781058850. [DOI] [PubMed] [Google Scholar]
- Damalas A., Kahan S., Shtutman M., Ben-Ze'ev A., Oren M. Deregulated beta-catenin induces a p53- and ARF-dependent growth arrest and cooperates with Ras in transformation. EMBO J. 2001;20:4912–4922. doi: 10.1093/emboj/20.17.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang D. T., Chen F., Gardner L. B., Cummins J. M., Rago C., Bunz F., Kantsevoy S. V., Dang L. H. Hypoxia-inducible factor-1alpha promotes nonhypoxia-mediated proliferation in colon cancer cells and xenografts. Cancer Res. 2006;66:1684–1936. doi: 10.1158/0008-5472.CAN-05-2887. [DOI] [PubMed] [Google Scholar]
- Deng J., Miller S. A., Wang H. Y., Xia W., Wen Y., Zhou B. P., Li Y., Lin S. Y., Hung M. C. beta-Catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- Deng J., Xia W., Miller S. A., Wen Y., Wang H. Y., Hung M. C. Crossregulation of NF-kappaB by the APC/GSK-3beta/beta-catenin pathway. Mol. Carcinog. 2004;39:139–146. doi: 10.1002/mc.10169. [DOI] [PubMed] [Google Scholar]
- Dikovskaya D., Schiffmann D., Newton I. P., Oakley A., Kroboth K., Sansom O., Jamieson T. J., Meniel V., Clarke A., Nathke I. S. Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis. J. Cell Biol. 2007;176:183–195. doi: 10.1083/jcb.200610099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Q., Zhang X., Cardinal J., Cao Z., Guo Z., Shao L., Geller D. A. Wnt/beta-catenin signaling regulates cytokine-induced human inducible nitric oxide synthase expression by inhibiting nuclear factor-kappaB activation in cancer cells. Cancer Res. 2009;69:3764–3771. doi: 10.1158/0008-5472.CAN-09-0014. [DOI] [PubMed] [Google Scholar]
- Fandrey J., Gorr T. A., Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc. Res. 2006;71:642–651. doi: 10.1016/j.cardiores.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Fearnhead N. S., Britton M. P., Bodmer W. F. The ABC of APC. Hum. Mol. Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- Furlan D., Sahnane N., Carnevali I., Cerutti R., Bertoni F., Kwee I., Uccella S., Bertolini V., Chiaravalli A. M., Capella C. Up-regulation of the hypoxia-inducible factor-1 transcriptional pathway in colorectal carcinomas. Hum. Pathol. 2008;39:1483–1494. doi: 10.1016/j.humpath.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Garcia J. A. HIFing the brakes: therapeutic opportunities for treatment of human malignancies. Sci. STKE. 2006;2006:pe25. doi: 10.1126/stke.3372006pe25. [DOI] [PubMed] [Google Scholar]
- Gorlach A., Bonello S. The cross-talk between NF-kappaB and HIF-1, further evidence for a significant liaison. Biochem. J. 2008;412:e17–19. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- Ichii S., et al. Detailed analysis of genetic alterations in colorectal tumors from patients with and without familial adenomatous polyposis (FAP) Oncogene. 1993;8:2399–2405. [PubMed] [Google Scholar]
- Kaidi A., Williams A. C., Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 2007;9:210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- Kenneth N. S., Mudie S., van Uden P., Rocha S. SWI/SNF regulates the cellular response to hypoxia. J. Biol. Chem. 2009;284:4123–4131. doi: 10.1074/jbc.M808491200. [DOI] [PubMed] [Google Scholar]
- Kenneth N. S., Rocha S. Regulation of gene expression by hypoxia. Biochem. J. 2008;414:19–29. doi: 10.1042/BJ20081055. [DOI] [PubMed] [Google Scholar]
- Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Nathke I. S. Tumor-associated NH2-terminal fragments are the most stable part of the adenomatous polyposis coli protein and can be regulated by interactions with COOH-terminal domains. Cancer Res. 2005;65:5195–5204. doi: 10.1158/0008-5472.CAN-04-4609. [DOI] [PubMed] [Google Scholar]
- Lim J. H., Chun Y. S., Park J. W. Hypoxia-inducible factor-1alpha obstructs a Wnt signaling pathway by inhibiting the hARD1-mediated activation of beta-catenin. Cancer Res. 2008;68:5177–5184. doi: 10.1158/0008-5472.CAN-07-6234. [DOI] [PubMed] [Google Scholar]
- Liu J., Stevens J., Rote C. A., Yost H. J., Hu Y., Neufeld K. L., White R. L., Matsunami N. Siah-1 mediates a novel beta-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol. Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- Liu X. W., Su Y., Zhu H., Cao J., Ding W. J., Zhao Y. C., He Q. J., Yang B. HIF-1alpha-dependent autophagy protects HeLa cells from fenretinide (4-HPR)-induced apoptosis in hypoxia. Pharmacol. Res. 2010 doi: 10.1016/j.phrs.2010.07.002. DOI 10.10.16/J.phrs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- McCartney B. M., Nathke I. S. Cell regulation by the Apc protein Apc as master regulator of epithelia. Curr. Opin. Cell Biol. 2008;20:186–193. doi: 10.1016/j.ceb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Menrad H., Werno C., Schmid T., Copanaki E., Deller T., Dehne N., Brune B. Roles of hypoxia-inducible factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of hepatocellular tumor spheroids. Hepatology. 2010;51:2183–2192. doi: 10.1002/hep.23597. [DOI] [PubMed] [Google Scholar]
- Metzen E., Stiehl D. P., Doege K., Marxsen J. H., Hellwig-Burgel T., Jelkmann W. Regulation of the prolyl hydroxylase domain protein 2 (phd2/egln-1) gene: identification of a functional hypoxia-responsive element. Biochem. J. 2005;387:711–717. doi: 10.1042/BJ20041736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E., Galluzzi L., Kepp O., Vicencio J. M., Criollo A., Maiuri M. C., Kroemer G. Anti- and pro-tumor functions of autophagy. Biochim. Biophys. Acta. 2009;1793:1524–1532. doi: 10.1016/j.bbamcr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Nathke I. S., Adams C. L., Polakis P., Sellin J. H., Nelson W. J. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J. Cell Biol. 1996;134:165–179. doi: 10.1083/jcb.134.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Byrne K. J., Dalgleish A. G., Browning M. J., Steward W. P., Harris A. L. The relationship between angiogenesis and the immune response in carcinogenesis and the progression of malignant disease. Eur. J. Cancer. 2000;36:151–169. doi: 10.1016/s0959-8049(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Perkins N. D., Gilmore T. D. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- Pescador N., Cuevas Y., Naranjo S., Alcaide M., Villar D., Landazuri M. O., Del Peso L. Identification of a functional hypoxia-responsive element that regulates the expression of the egl nine homologue 3 (egln3/phd3) gene. Biochem. J. 2005;390:189–197. doi: 10.1042/BJ20042121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps R. A., Chidester S., Dehghanizadeh S., Phelps J., Sandoval I. T., Rai K., Broadbent T., Sarkar S., Burt R. W., Jones D. A. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Rajaganeshan R., Prasad R., Guillou P. J., Poston G., Scott N., Jayne D. G. The role of hypoxia in recurrence following resection of Dukes' B colorectal cancer. Int. J. Colorectal Dis. 2008;23:1049–1055. doi: 10.1007/s00384-008-0497-x. [DOI] [PubMed] [Google Scholar]
- Rius J., Guma M., Schachtrup C., Akassoglou K., Zinkernagel A. S., Nizet V., Johnson R. S., Haddad G. G., Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha S., Garrett M. D., Campbell K. J., Schumm K., Perkins N. D. Regulation of NF-kappaB and p53 through activation of ATR and Chk1 by the ARF tumour suppressor. EMBO J. 2005;24:1157–1169. doi: 10.1038/sj.emboj.7600608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom O. J., et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm K., Rocha S., Caamano J., Perkins N. D. Regulation of p53 tumour suppressor target gene expression by the p52 NF-kappaB subunit. EMBO J. 2006;25:4820–4832. doi: 10.1038/sj.emboj.7601343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. T., Colgan S. P. Hypoxia and gastrointestinal disease. J. Mol. Med. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- van Uden P., Kenneth N. S., Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem. J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S., Ryan K. M. Growth factor signaling permits hypoxia-induced autophagy by a HIF1alpha-dependent, BNIP3/3L-independent transcriptional program in human cancer cells. Autophagy. 2009;5:1068–1069. doi: 10.4161/auto.5.7.9821. [DOI] [PubMed] [Google Scholar]
- Yoo Y. G., Hayashi M., Christensen J., Huang L. E. An essential role of the HIF-1alpha-c-Myc axis in malignant progression. Ann. NY Acad. Sci. 2009;1177:198–204. doi: 10.1111/j.1749-6632.2009.05043.x. [DOI] [PubMed] [Google Scholar]
- Yu L., Strandberg L., Lenardo M. J. The selectivity of autophagy and its role in cell death and survival. Autophagy. 2008;4:567–573. doi: 10.4161/auto.5902. [DOI] [PubMed] [Google Scholar]
- Zhang H., Bosch-Marce M., Shimoda L. A., Tan Y. S., Baek J. H., Wesley J. B., Gonzalez F. J., Semenza G. L. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.