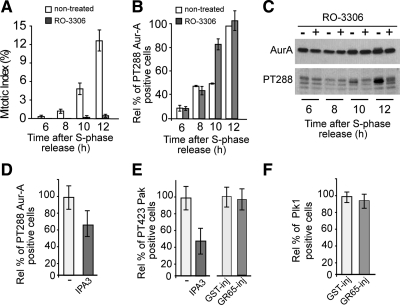
Figure 5.
Block of Golgi fragmentation inhibits Aur-A recruitment through a novel mechanism. HeLa cells were grown on coverslips and arrested in S phase by using the double-thymidine block. (A–C) Four hours after S phase block release, the cells were either left in growth medium (nontreated) or treated with 9 μM RO-3306 (a Cdk1 inhibitor) and fixed and processed for immunofluorescence under confocal microscopy or immunoblotting at the indicated times after thymidine removal. Cells were labeled with Hoechst 33342 to determine the mitotic indices up to 12 h after S phase release (A) and with antibodies against T288-phosphorylated Aur-A to determine the relative percentages of pT288 Aur-A–positive cells calculated according to the nontreated cells fixed up to 12 h after S phase release (B). A similar set of samples was processed for immunoblotting to reveal total Aur-A and active Aur-A (PT288) (C). (D–F) Cells were either nonmicroinjected (D and E) or microinjected 1 h after thymidine washout with recombinant GST (GST-inj; 8 mg/ml) or recombinant GRASP65 (GR65-inj, 8–10 mg/ml; E and F), and with FITC-conjugated dextran as microinjection marker (E and F). Eight hours after S phase block release, the nonmicroinjected cells were either left in growth medium (D and E, −) or treated with 30 μM IPA3 (D and E, a Pak inhibitor) and fixed 12 h after the S-phase block release for immunofluorescence under confocal microscopy with antibodies against T288-phosphorylated Aur-A (D), against T423-phosphorylated Pak (E), or against Plk1 (F). The relative percentages of PT288 Aur-A-positive (D), T423 Pak-positive (E) were calculated according to the relevant cells treated with vehicle (−). The relative percentages of Plk1-positive cells (F) were calculated according the noninjected cells on the same coverslip. Quantification data are means ± SD from at least two independent experiments, each carried out in duplicate. More than 200 cells were microinjected for each condition.