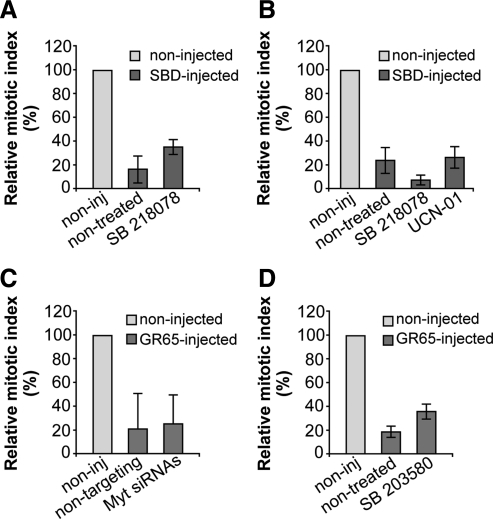
Figure 9.
The known G2 checkpoints are not involved in the Golgi-dependent block of the cell cycle. HeLa cells (A, C, and D) and NRK cells (B) were grown on coverslips and left untreated (C) or arrested in S phase by using the double-thymidine block. (A and B) One hour after S phase block release, the cells were microinjected (or not; noninj) with recombinant SBD (8–12 mg/ml, SBD-inj) and FITC-conjugated dextran as microinjection marker. Next, 4 h after S phase block release the cells were either left untreated or treated with 2.5 μM SB218078 or 300 nM UCN-01. The cells were fixed 12 h after the final thymidine washout and processed for immunofluorescence under confocal microscopy. (C) The cells were transfected after the first thymidine block of the cell synchronization protocol, for 24 h with 20 nM nontargeting siRNAs or with 20 nM anti-Myt1 siRNAs, or processed without siRNAs transfection. One hour after S phase block release, cells were microinjected (or not; noninj) with recombinant GRASP65 (8–10 mg/ml), and FITC-conjugated dextran as microinjection marker. (D) One hour after S phase block release, cells were microinjected (or not; noninj) with recombinant GRASP65 (8–10 mg/ml), and FITC-conjugated dextran as microinjection marker. After 4 h without thymidine, cells were either left nontreated or treated with 10 μM SB203580 (a p38 kinase inhibitor). For all quantification data (A–D), the cells were fixed 12 h after the second thymidine washout, processed for immunofluorescence, and observed under confocal microscopy, with labeling with Hoechst 33342 for cell cycle phase. Quantification of the relative mitotic indices is shown as percentages of microinjected cells in mitosis normalized to nonmicroinjected cells on the same coverslip. More than 200 cells were microinjected for each condition, and data are means ± SD from two (B) or three (A, C, and D) independent experiments, each carried out in duplicate.