Abstract
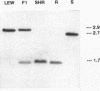
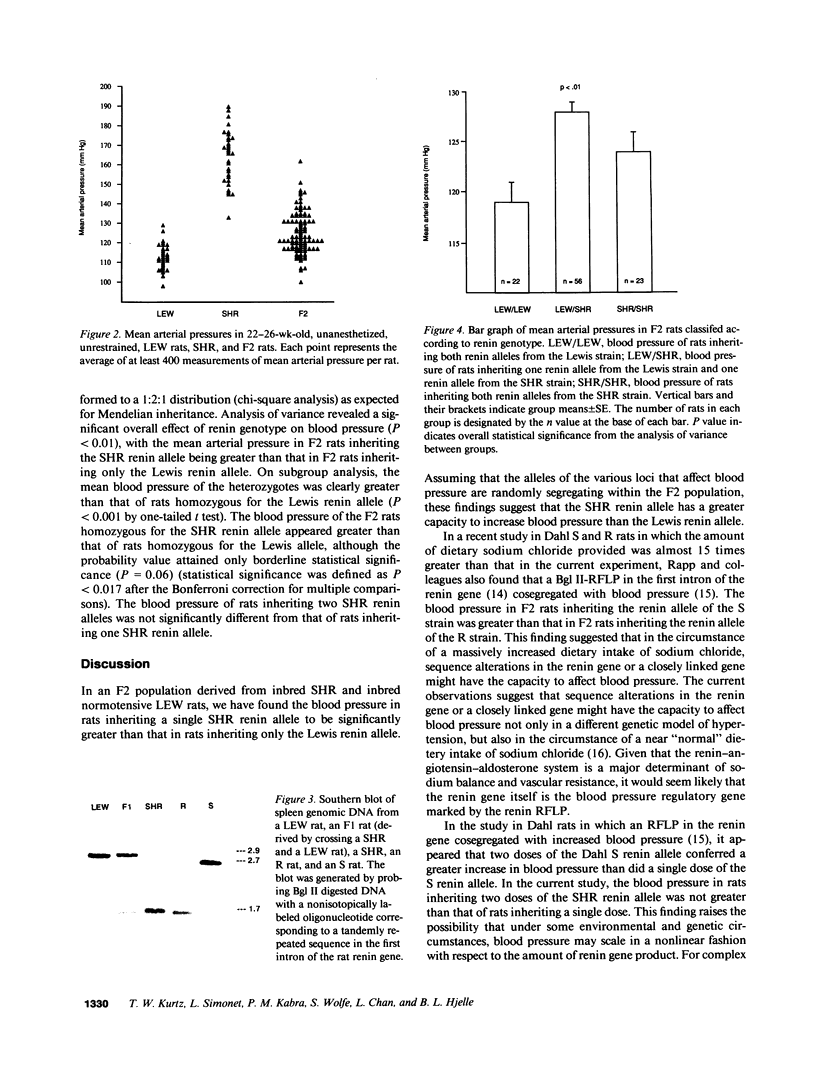
The spontaneously hypertensive rat (SHR) exhibits alterations in the renin-angiotensin-aldosterone system which are similar to those that characterize patients with "nonmodulating" hypertension, a common and highly heritable form of essential hypertension. Accordingly, we determined whether the inheritance of a DNA restriction fragment length polymorphism (RFLP) marking the renin gene of the SHR was associated with greater blood pressure than inheritance of a RFLP marking the renin gene of a normotensive control rat. In an F2 population derived from inbred SHR and inbred normotensive Lewis rats, we found the blood pressure in rats that inherited a single SHR renin allele to be significantly greater than that in rats that inherited only the Lewis renin allele. To the extent that the SHR provides a suitable model of "nonmodulating" hypertension, these findings raise the possibility that a structural alteration in the renin gene, or a closely linked gene, may be a pathogenetic determinant of increased blood pressure in one of the most common forms of essential hypertension in humans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagby S. P., McDonald W. J., Mass R. D. Serial renin-angiotensin studies in spontaneously hypertensive and Wistar-Kyoto normotensive rats. Transition from normal- to high-renin status during the established phase of spontaneous hypertension. Hypertension. 1979 Jul-Aug;1(4):347–354. doi: 10.1161/01.hyp.1.4.347. [DOI] [PubMed] [Google Scholar]
- Dzau V. J., Burt D. W., Pratt R. E. Molecular biology of the renin-angiotensin system. Am J Physiol. 1988 Oct;255(4 Pt 2):F563–F573. doi: 10.1152/ajprenal.1988.255.4.F563. [DOI] [PubMed] [Google Scholar]
- Fukamizu A., Nishi K., Cho T., Saitoh M., Nakayama K., Ohkubo H., Nakanishi S., Murakami K. Structure of the rat renin gene. J Mol Biol. 1988 May 20;201(2):443–450. doi: 10.1016/0022-2836(88)90151-9. [DOI] [PubMed] [Google Scholar]
- Guidi E., Hollenberg N. K. Differential pressor and renal vascular reactivity to angiotensin II in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 1987 Jun;9(6):591–597. doi: 10.1161/01.hyp.9.6.591. [DOI] [PubMed] [Google Scholar]
- Harrap S. B., Doyle A. E. Genetic co-segregation of renal haemodynamics and blood pressure in the spontaneously hypertensive rat. Clin Sci (Lond) 1988 Jan;74(1):63–69. doi: 10.1042/cs0740063. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J. Highly variable minisatellites and DNA fingerprints. Biochem Soc Trans. 1987 Jun;15(3):309–317. doi: 10.1042/bst0150309. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kawabe K., Watanabe T. X., Shiono K., Sokabe H. Influence on blood pressure of renal isografts between spontaneously hypertensive and normotensive rats, utilizing the F1 hybrids. Jpn Heart J. 1978 Nov;19(6):886–894. doi: 10.1536/ihj.19.886. [DOI] [PubMed] [Google Scholar]
- Kitami Y., Hiwada K., Kokubu T. Kidney renin gene expression in spontaneously hypertensive rats. J Hypertens. 1989 Sep;7(9):727–731. [PubMed] [Google Scholar]
- Kurtz T. W., Montano M., Chan L., Kabra P. Molecular evidence of genetic heterogeneity in Wistar-Kyoto rats: implications for research with the spontaneously hypertensive rat. Hypertension. 1989 Feb;13(2):188–192. doi: 10.1161/01.hyp.13.2.188. [DOI] [PubMed] [Google Scholar]
- Kurtz T. W., Morris R. C., Jr Hypertension in the recently weaned Dahl salt-sensitive rat despite a diet deficient in sodium chloride. Science. 1985 Nov 15;230(4727):808–810. doi: 10.1126/science.4059913. [DOI] [PubMed] [Google Scholar]
- Laragh J. H. Vasoconstriction-volume analysis for understanding and treating hypertension: the use of renin and aldosterone profiles. Am J Med. 1973 Sep;55(3):261–274. doi: 10.1016/0002-9343(73)90128-9. [DOI] [PubMed] [Google Scholar]
- Lifton R. P., Hopkins P. N., Williams R. R., Hollenberg N. K., Williams G. H., Dluhy R. G. Evidence for heritability of non-modulating essential hypertension. Hypertension. 1989 Jun;13(6 Pt 2):884–889. doi: 10.1161/01.hyp.13.6.884. [DOI] [PubMed] [Google Scholar]
- OKAMOTO K., AOKI K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963 Mar;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- Rapp J. P., Wang S. M., Dene H. A genetic polymorphism in the renin gene of Dahl rats cosegregates with blood pressure. Science. 1989 Jan 27;243(4890):542–544. doi: 10.1126/science.2563177. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Blumenfeld J. D., Bell G. M., Pecker M. S., Sommers S. C., Laragh J. H. On the renal basis for essential hypertension: nephron heterogeneity with discordant renin secretion and sodium excretion causing a hypertensive vasoconstriction-volume relationship. J Hypertens. 1988 Oct;6(10):763–777. doi: 10.1097/00004872-198811000-00001. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Rubattu S. Prorenin and renin as separate mediators of tissue and circulating systems. Am J Hypertens. 1989 May;2(5 Pt 1):358–366. doi: 10.1093/ajh/2.5.358. [DOI] [PubMed] [Google Scholar]
- Shoback D. M., Williams G. H., Moore T. J., Dluhy R. G., Podolsky S., Hollenberg N. K. Defect in the sodium-modulated tissue responsiveness to angiotensin II in essential hypertension. J Clin Invest. 1983 Dec;72(6):2115–2124. doi: 10.1172/JCI111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanase H., Suzuki Y., Ooshima A., Yamori Y., Okamoto K. Genetic analysis of blood pressure in spontaneously hypertensive rats. Jpn Circ J. 1970 Dec;34(12):1197–1212. doi: 10.1253/jcj.34.1197. [DOI] [PubMed] [Google Scholar]
- Wang S. M., Rapp J. P. Structural differences in the renin gene of Dahl salt-sensitive and salt-resistant rats. Mol Endocrinol. 1989 Feb;3(2):288–294. doi: 10.1210/mend-3-2-288. [DOI] [PubMed] [Google Scholar]
- Williams G. H., Braley L. M., Menachery A. Decreased adrenal responsiveness to angiotensin II: a defect present in spontaneously hypertensive rats. A possible model of human essential hypertension. J Clin Invest. 1982 Jan;69(1):31–37. doi: 10.1172/JCI110438. [DOI] [PMC free article] [PubMed] [Google Scholar]