Abstract
Respiratory Syncytial Virus (RSV) is the leading cause of pneumonia and bronchiolitis in infants and children <1 year old, resulting in significant morbidity and mortality worldwide. There is currently no RSV vaccine. In the 1960’s, a formalin-inactivated RSV (FI-RSV) vaccine trial led to exacerbated disease upon natural infection of vaccinees, including two deaths. The causes involved in the disastrous results of these vaccine trials are still unclear but they remain the engine for searching new avenues to develop a safe vaccine that can provide long-term protection against this important pathogen. This article reviews some of the early history of RSV vaccine development, as well as more recent information on the interaction between RSV and the host innate and adaptive immune responses. A safe and efficacious vaccine for RSV will require “re-education” of the host immune response against RSV to prevent vaccine-enhanced or severe RSV disease.
Keywords: RSV vaccine, TLR-4, vaccine enhancement of disease
INTRODUCTION
RSV, the most significant cause of serious lower respiratory tract infection in infants and young children1, results in 75,000–125,000 hospitalizations2 and ~500 deaths yearly in the USA3. Due to the horrific outcome of a failed clinical trial in the mid-1960’s in which a formalin-inactivated RSV (FI-RSV) vaccine preparation was evaluated (discussed below), interest in live attenuated vaccines followed. In a recent review, Schickli et al.4 have provided an in depth analysis of the constraints associated with the development of live attenuated vaccines for RSV. They note that since the most vulnerable population is infants, an ideal RSV vaccine would have to be administered very early in life, provide protection in the presence of maternal antibody, not interfere with the safety or efficacy of other vaccines routinely administered to infants (and vice versa), and elicit protection with minimal reactogenicity. In addition, we believe that any licensed RSV vaccine will have to be readily accepted by parents and would likely need to elicit immunity that is more durable than that provided by natural infection, a unique requirement for a vaccine. All of these constraints apply to subunit vaccines as well. In this chapter, we will focus on the immunological aspects of developing a safe and efficacious RSV vaccine, with particular emphasis on the roles of RSV fusion (F) protein and Toll-like receptor 4 (TLR4) in this process.
Respiratory Syncytial Virus (RSV): A long-term challenge to vaccine development
Although severe RSV-induced disease predominates in infants and children, it has more recently been identified as an increasing cause of morbidity and mortality in the elderly5–7, transplant patients, and in patients with immunodeficiencies8, 9. RSV is relatively stable antigenically, yet most adults are re-infected every few years, suggesting that natural immunity is not long-lasting10. Why we fail to develop durable immunity after RSV infection is not understood. It is also unclear why children, the elderly, and the immunosuppressed are at a much higher risk for severe disease; however, an immune pathological mechanism has long been suspected in the development of severe RSV disease. In children, severe RSV disease is most often associated with prematurity, bronchopulmonary dysplasia (BPD), or congenital heart disease (CHD)11. RSV-specific immunity has been implicated in both protection and the immunopathological mechanism(s) that lead to severe lower respiratory tract disease and long-term changes in the immunological environment of the lung, i.e., RSV infection in early infancy has been correlated with development of allergic and asthmatic symptoms later in life12. While there is currently no licensed RSV vaccine, the two antibodies licensed for prophylactic use, RespiGam® and Synagis®, unquestionably provide highly significant passive protection to high-risk infants13, 14. However, due to the high cost of antibody prophylaxis, the USA is the only country that administers this drug to the majority of high-risk infants. Therefore, in the absence of a safe and effective vaccine, antibody prophylaxis and parental education, including basic hygiene practices on avoiding RSV, are the only options for reducing infection in healthy infants, children, and adults15, populations with the majority of RSV-related hospitalizations.
The idea that the immune response plays an adverse role in RSV-induced disease is based largely on the observations from an early clinical trial in which vaccination of infants with formalin-inactivated RSV (FI-RSV) resulted in greatly enhanced susceptibility to develop severe lower respiratory tract involvement upon natural RSV infection. Thus, a better understanding of the immune mechanisms operative in primary, secondary, and vaccine-enhanced infection will be required to identify novel approaches to induce safe, long-lasting immunity to RSV and/or to intervene therapeutically.
RSV, a member of the family Paramyxoviridae, has a nonsegmented negative strand genome11. The virion consists of a nucleocapsid within a lipid envelope of irregular size and shape. Three glycoproteins are found in the virus envelope. The hydrophobic SH protein is the smallest and its function is relatively unknown. Fusion (F) protein mediates viral penetration and cell to cell spread by fusion of membranes. Fusion of RSV with host cell membranes is required for transfer of the viral ribonucleoprotein into the cell cytoplasm. F protein also promotes syncytia formation due to fusion of infected cell membranes with those of adjacent cells. This phenomenon may have limited relevance, however, since it is often seen in vitro, but infrequently in vivo. RSV F protein shares structural elements and a low, but significant, level of sequence homology with other paramyxoviruses16. RSV F protein contains N-linked oligosaccharide groups and is composed of two disulfide-linked protein subunits of 47 (F1) and 20 kD (F2). The G protein mediates virus attachment. Two major antigenic groups, A and B, are distinguished primarily by differences in the G protein, with group A being most prevalent. G and SH proteins are not required for infectivity, as demonstrated by the isolation of a viable RSV mutant (cp52) lacking these genes17. Conversely, RSV mutants that lack the F protein gene cannot replicate. The F protein is highly conserved among the two antigenic groups of RSV and has long been recognized as a major vaccine candidate as it is an immunodominant target for neutralizing antibodies18 and for virus-specific cytotoxic T lymphocytes19. A passively administered humanized (IgG1, κ) monoclonal antibody (MAb) (i.e., Synagis®) that completely protected cotton rats (Sigmodon hispidus) against RSV challenge20, significantly reduces disease severity in high-risk infants14. The neutralizing epitope that is recognized by Synagis® is contained within the F protein21.
Failed RSV human vaccine trials
Shortly after RSV isolation, NIH initiated a program to develop a vaccine using an approach that had been successfully applied to polio and influenza. Based on the prior success of other formalin-inactivated virus vaccines, a similar procedure was attempted for RSV. RSV "Lot 100” vaccine was propagated in monkey kidney cells, formalin-inactivated, and concentrated by ultracentrifugation and alum precipitation. Clinical trials began in winter of 1965–1966 in 4 centers in the USA. The results of these trials were entirely unexpected22–25: there was no protection against RSV in vaccinees whose rate of naturally occurring infection was significantly higher than in controls. Second, RSV infection caused more severe disease in vaccinees, with a 16-fold increase in hospitalizations and two fatalities among the youngest patients, who likely had no previous natural exposure to RSV. The legacy of Lot 100 has had a profoundly negative influence on vaccine development, and no RSV vaccine has since been licensed for any age group.
Analysis of the failed vaccine trials and early epidemiological studies yielded two intriguing correlations. First, those Lot 100 vaccinees who developed enhanced RSV disease had significant levels of serum antibody to RSV at the time of illness. In contrast, parainfluenza-vaccinated or unvaccinated controls experienced relatively mild RSV infection and had much lower titers of anti-RSV antibody22–25. Second, severe RSV disease was observed most frequently in infants <6 months old, when maternal antibody is present26. The lack of an animal model at the time of the Lot 100 trials precluded experimental corroboration. Nonetheless, these observations led to the hypothesis that antibody, normally considered protective, contributed to RSV disease severity. However, the subsequent finding that prophylactic administration of polyclonal human anti-RSV immunoglobulin or anti-RSV F protein MAb to cotton rats is both safe and protective against primary RSV disease26–29, diverted attention away from antibodies as mediators of RSV-induced disease and led to the highly successful prophylactic use of polyclonal, and subsequently MAb, anti-RSV antibodies in high-risk infants.
After the failed FI-RSV trial, concerns over safety prompted development of live attenuated vaccines with cold-passaged (cp), temperature-sensitive (ts) mutations or recombinant virus with deletion mutations (SH, NS1 or NS2) combined with the aforementioned (reviewed in4). Such vaccines exhibited residual virulence, genetic instability, or insufficient immunogenicity in clinical testing30, 31. Subunit vaccines (e.g., chromatographically purified F glycoprotein32–34, recombinant chimeric F/G glycoprotein35, recombinant chimeric RSV-F/parainfluenza–HN glycoprotein36, plasmid DNA encoding F and G glycoproteins37–41, recombinant G glycoprotein42, 43, and G protein peptides44, 45 were also developed. However, since the failed FI-RSV vaccine trial, no non-replicating RSV vaccine has been put into clinical trials in immunologically naïve infants, and it is likely that approval for any future trial will be contingent upon demonstrating a compelling safety profile for candidate vaccines in animal models.
In 1971, the susceptibility of the cotton rat S. hispidus to human RSV was described46. Human RSV replicates to high titers in the nose and lungs of cotton rats of all ages and they are 50–1000-times more permissive than several inbred mouse strains47. Viral antigen can be detected in the nasal, bronchial, and bronchiolar epithelium48. Primary RSV infection in S. hispidus lasts ~5 days in the lungs and slightly longer in the nose. Lower doses cause mild to moderate peribronchiolitis (inflammatory cells, primarily lymphocytes, around the small airways), while ≥106 plaque-forming units (pfu) also cause interstitial pneumonitis (thickening of alveolar walls accompanied by inflammatory cells) and alveolitis (inflammatory cells in air spaces), compromising pulmonary function.
Passive administration of polyclonal anti-RSV antibody prophylactically was first shown to be protective in cotton rats49, and then in humans13, 14, 50. These findings led to licensure of RespiGam® and, later, the MAb anti-F protein antibody, Synagis®, for prevention of severe RSV disease in high-risk infants. Both products advanced to human clinical trials on the strength of data from cotton rat studies alone. Prince et al. reproduced vaccine-enhanced disease by immunizing cotton rats with the original Lot 100 vaccine, followed by in. RSV challenge51. Although most mouse strains are less susceptible to RSV than cotton rats, certain inbred mouse strains52, or mice that lack genes that encode key inflammatory molecules (e.g., TLR4, TLR2, STAT1, STAT6, IFN-γ, IL-13, and IL-4Rα43, 53–58), have provided important insights into mechanisms of immunity to RSV. Among inbred mouse strains, the BALB/c strain appears to be more sensitive than other commonly used inbred mouse strains, e.g., C57BL/6, although high inocula, compared to cotton rats, are required to elicit severe pathology59.
Molecular insights into potential mechanisms of enhanced disease
Prince et al.51 identified 2 peaks of pulmonary disease following RSV challenge of FI-RSV-immunized cotton rats. The timing of the first peak (1 day p.i.) and the predominant infiltrating cell type (neutrophils) were suggestive of antigen-antibody and complement deposition, similar to a type III "Arthus reaction." A second peak of pulmonary disease (4 days p.i.) seen in cotton rats with vaccine-enhanced disease is associated with a predominance of lymphocytes, suggesting a delayed, cell-mediated immune mechanism51. Enhanced disease in the cotton rat60 presents with alveolitis, consisting primarily of neutrophil infiltrates, and peribronchiolitis, consisting primarily of lymphocyte infiltrates, features that are remarkably similar to the original histopathology described for the two Lot 100 fatalities61. The fact that alveolitis is a hallmark of FI-RSV underscores its importance as a histologic marker of vaccine-enhanced disease. When the results of the failed vaccine trial were published and the results of the two autopsies described, Kim et al.25 commented only briefly on the histopathologic findings, citing “peribronchiolar monocytic infiltration with some excess in eosinophils.” Unfortunately, this description did not accurately reflect the original autopsy reports. Kim et al.’s mention of eosinophils, and failure to mention neutrophils, have led many to conclude incorrectly that eosinophilia is a primary marker of vaccine-enhanced RSV disease (e.g.,62–64), whereas neutrophilia was, in fact, the predominant histologic finding of both autopsies61. Vaccine-enhanced RSV disease in African green monkeys65 and calves66 is also characterized by neutrophilic alveolitis, without eosinophils. In contrast, enhanced disease in mice is not accompanied by neutrophils67, and pulmonary eosinophilia, while predominant in some strains of mice (e.g., BALB/c), is absent in others52.
Graham et al.68 first reported that vaccine-enhanced disease was associated with a “Th2 type” response. BALB/c mice immunized with FI-RSV, then challenged with RSV, exhibited a pattern of cytokine mRNA expression characterized by an increased ratio of IL-4/IFN-γ. In contrast, unvaccinated animals showed a “Th1 type” response to RSV infection, with undetectable IL-468. In some mouse strains, this Th2 type response was accompanied by pulmonary eosinophilia and increased CD4+ T cells56, 62–64, 69. Although an imbalance in cytokine levels favoring a Th2 “skew” has frequently been put forth to explain FI-RSV-induced vaccine-enhanced disease, it has not been possible to corroborate this in humans since cytokine levels were not measured in the original FI-RSV trial. A number of studies have investigated cytokine profiles in supernatants of peripheral blood mononuclear cells and nasopharyngeal aspirates of patients with RSV and correlated these findings with disease severity70–78. Although a direct comparison of these studies is difficult because of differences in the cohorts used and the endpoints considered, increases in Th2-like responses in infants with severe RSV disease were reported in some studies70, 71, 74, 75, 77, whereas other similar studies reported induction of both Th1 and Th2 type cytokines73 or no evidence of a Th1-Th2 cytokine imbalance72, 76, 78. Finally, inflammatory mediators in plasma, inflammatory cells in nasal washings, and virus-specific responses in T cell cultures established from peripheral blood mononuclear cells of infants <6 months were analyzed in relation to clinical severity. IL-6 and IL-8 were found more frequently and at higher levels in plasma samples of more severely ill patients, but no significant differences were found in the levels of cytokines that distinguish Th1 and Th2 responses79. These conflicting results raise the possibility that Th2 cytokines may be more readily detected in FI-RSV-enhanced disease than in primary RSV infection. It is also possible that the mechanism(s) underlying FI-RSV-enhanced disease differ(s) from that of severe primary infection, despite the frequent implication in the literature that they are similarly mediated.
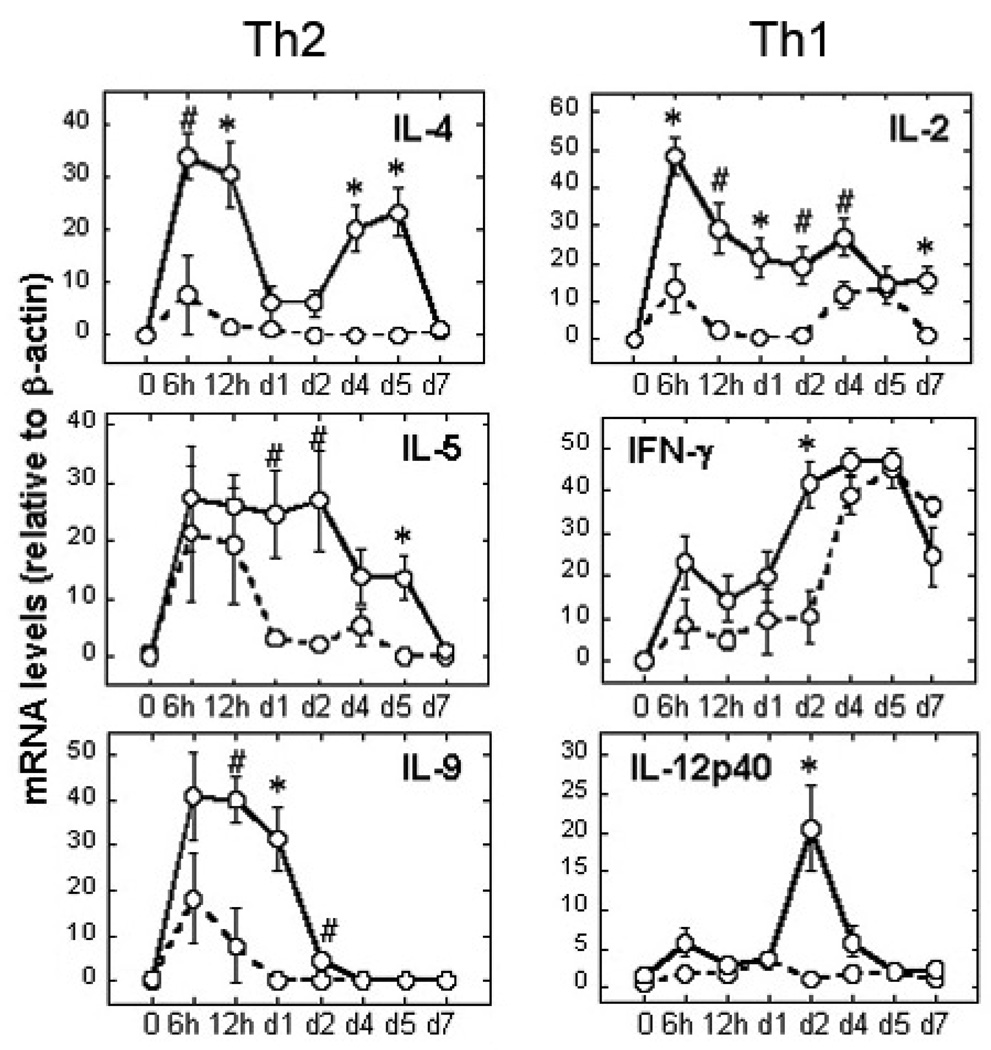
Using the identical Lot 100 FI-RSV vaccine from the failed clinical trials, we previously established a cotton rat model for enhanced RSV disease that faithfully recapitulated the pathology induced by RSV infection in Lot 100-immunized children51, 61. Therefore, we sought to characterize FI-RSV-enhanced pathology molecularly, again using the original Lot 100 vaccine80. We postulated that FI-RSV vaccine-enhanced disease was due to a failure of formalin-modified RSV F protein to trigger a strong proinflammatory “Th1” response due to a diminished capacity for interaction with TLR4, resulting in a “Th2”-type bias81. Using reverse transcription (RT)-PCR, expression of 19 genes (e.g., pro- and anti-inflammatory cytokines, chemokines, and others) was quantified after RSV challenge of animals vaccinated with a mock vaccine vs. Lot 100 FI-RSV. We confirmed that FI-RSV-enhancement of RSV-mediated disease was associated with a significant increase in neutrophilic alveolitis, a marker of vaccine-enhanced disease both in cotton rats and infants61. The expression patterns of several cytokine/chemokine mRNAs differed dramatically between RSV-challenged, mock- vs. FI-RSV-vaccinated cotton rats (Figure. 1). FI-RSV-enhanced disease was associated with a stronger cytokine response than that seen in primary infection controls (mock-vaccinated animals). For example, vaccination with FI-RSV resulted in statistically significant increases in mRNA levels for Th2 markers, IL-4 and IL-5. IL-9, considered to be a key Th2 cytokine involved in asthma predisposition82, was also highly elevated in FI-RSV-vaccinated animals after RSV infection, supporting previous results in other animal models83.
Fig. 1.
Lung cytokine gene expression during FI-RSV vaccine-enhanced disease. Cotton rats were vaccinated with mock- or FI-RSV vaccine (days 0 and 28). Four weeks later, animals were challenged with RSV and sacrificed at the indicated times. mRNA levels of prototype Th2 and Th1 cytokine genes were quantified by RT-PCR, and normalized to β-actin. Results represent the mean ± S .E.M. of 6–8 animals per time point for FI-RSV-vaccinated animals (solid line) and 4–6 animals per time point for mock-vaccinated (dotted line). #, p < 0.05; *, p < 0.01; (FI-RSV- vs. mock-vaccinated animals). This figure represents a subset of 19 genes examined. Adapted from reference80.
Surprisingly, the effect of FI-RSV vaccination on gene induction by RSV infection was not restricted to an upregulation of Th2 cytokines as has been perpetuated in the literature based on early studies in the murine (BALB/c) model of RSV infection68. Chemokine expression was also increased significantly, e.g., Monocyte Chemoattractant Protein-1 (MCP-1) mRNA was dramatically increased only in FI-RSV-vaccinated animals early after infection80. In addition, the expression of genes that encode prototypical Th1 cytokines, e.g., IL-2, IFN-γ, and IL-12 p40, were significantly increased in RSV-infected cotton rats that were previously vaccinated with FI-RSV (Fig. 1, Th1). Collectively, our results challenge the long-standing paradigm that a skew toward Th2-type cytokines underlies FI-RSV-induced enhancement of RSV disease. Our data indicate a more complex and generalized dysregulation of the immune response revealed by a significant enhancement of not only Th2 cytokines, but also Th1-type cytokines and chemokines not previously associated with RSV infection of FI-RSV-sensitized animals.
We also found that enhanced disease, normally associated with FI-RSV, could be induced by a vaccination of cotton rats against human metapneumovirus (hMPV), a paramyxovirus related to RSV84. Cotton rats vaccinated with formalin-inactivated (FI)-hMPV and challenged with hMPV exhibit dramatic enhancement of lung pathology. In contrast to RSV, FI-hMPV-enhanced pathology is paralleled by increased IL-4 and a decrease in IFN-γ. Thus, vaccine-enhanced disease induced by different FI-paramyxovirus vaccines cannot be characterized only by changes in Th1/Th2 ratio. Individual components of each vaccine, or the manner in which live virus subsequently engages primed host cells, likely activate the innate and adaptive immune responses to infection in mechanistically distinct ways.
Role of Toll-like receptor 4 (TLR4) in the response to lipopolysaccharide (LPS) and RSV
LPS, an integral outer membrane component of all Gram negative bacteria, is one of the most potent inflammatory stimuli. LPS activates macrophages and other cells to produce TNF-α, IL-1β, and other potent cytokines and chemokines that induce inflammation through receptor-mediated interactions with target cells. LPS signaling is initiated when circulating LPS-binding protein (LBP) transfers LPS to CD14. CD14 exists on the surface of certain cell types, e.g., macrophages, as a glycosyl phosphatidylinositol (GPI)-linked protein, or in a soluble form (sCD14). Both forms of CD14 bind monomeric LPS with high affinity, and transfer it to MD-2, an extracellular protein that binds to the TLR4 ectodomain. CD14 does not signal intracellularly because it has no intracellular portion with which to transmit intracellular signals. CD14−/− mice, and their macrophages, are poorly LPS-responsive85, 86.
The discovery of TLR4 proved central in our understanding of LPS-induced signaling. TLR4 is one of >10 human homologs of Drosophila Toll, a transmembrane, signaling molecule required for innate immunity to fungal and bacterial infection in adult flies. Ligand-mediated dimerization of TLRs leads to intracellular signaling. Purified enterobacterial LPS preparations utilize TLR4 exclusively for signaling. In contrast, TLR2 responds to bacterial lipopeptides but, rather than forming homodimers, TLR2 signaling requires formation of heterodimers between TLR2 and either TLR1 or TLR6. TLR5 senses bacterial flagellin. While TLRs 2/1, 2/6, 4, and 5 are typically associated with detection of extracellular microbes through their N-terminal ectodomains, TLRs 3, 7, 8, and 9 are found in endosomes where they detect viral or bacterial nucleic acids (reviewed in87). Critical to the establishment of TLR4 as the LPS signal transducer was the finding that certain spontaneously derived, LPS-hyporesponsive inbred mouse strains possess TLR4 mutations or deletions88–90, later confirmed in TLR4−/− mice (reviewed in91). Macrophages from mice with tlr4 mutations, or from TLR4−/− mice, fail to respond to LPS with inflammatory gene expression92. MD-2, a non-membrane-spanning protein that associates with the TLR4 ectodomain, is required for LPS-induced, TLR4 signaling93. Close physical proximity of LPS and TLR4 is likely achieved by CD14-mediated transfer of LPS to MD-294 or the generation of stable LPS:MD-2 complexes95 that lead to TLR4 dimerization, followed by recruitment of downstream signaling molecules. Recently, the crystal structures of murine and human MD-2 were solved96, 97. The structural basis of LPS recognition by the TLR4- MD-2 complex98 was recently resolved and revealed that MD-2 binds to the concave surface of the N-terminal and central domains of TLR4 and that the acyl chains of the lipid A region of LPS insert into a deep hydrophobic pocket in MD-2, thereby inducing heterotetramer formation (i.e., (TLR4/MD-2)2).
Three domains, i.e., a Leucine Rich Region (LRR) in the N-terminal ectodomain, a transmembrane region, and a Toll/IL-1R resistance (TIR) domain in the intracellular region, are structural hallmarks of all known TLRs (reviewed in91). While the overall structure of different TLRs is similar, the pattern of gene expression induced by TLR4 differs substantially from that induced by other TLRs, such as TLR299 or TLR9100. TLR4, but not TLR2, activation results in induction of IFN-β, that, in turn, acts in an autocrine/paracrine fashion to activate STAT199. Many genes not induced by TLR2 agonists (e.g., IP-10, MCP-5, and iNOS) are STAT1-dependent101. Differential utilization of 4 TIR-containing adapter molecules (i.e., MyD88, TIRAP, TRIF, and TRAM) by distinct TLRs leads to activation of distinct downstream signaling pathways, findings based largely on studies in adapter knockout mice. Two major TLR signaling pathways were identified, i.e., one that is MyD88-dependent, and gives rise to strong and early activation of NF-κB, and a TRIF-dependent, MyD88-independent pathway that primarily drives strong activation of another key transacting factor, IRF-3, with delayed NF-κB. The MyD88-dependent pathway results in induction of highly NF-κB-dependent, proinflammatory genes (e.g., TNF-α, IL-1β), while the MyD88-independent pathway leads to IRF-3-dependent gene induction (e.g., IFN-β, RANTES). TLR4 is unique in that it activates both pathways for gene expression because it is the only TLR that uses all 4 adapter proteins102.
Innate immunity, initiated by the interaction of host cells with pathogens through recognition of conserved microbial structures, is central in the early response to infection and facilitates development of the adaptive immune response. Kurt-Jones and colleagues first reported that the RSV F protein triggers the innate immune response to RSV through mammalian TLR4 and CD1453, 103. Although RSV F protein shares no structural similarity with LPS, it requires CD14, TLR453, 104 and MD-2 (manuscript in preparation) to signal. Importantly, the ability to clear live RSV was impaired in mice with tlr4 mutations53, 103, indicating that TLR4 signaling is necessary for control of primary RSV replication. Haynes et al.103 found that TLR4-deficient mice challenged with RSV also exhibited impaired NK and CD14+ cell pulmonary trafficking, diminished NK cell function, and impaired IL-12 induction, in addition to impaired RSV clearance. Haeberle et al.105 employed a model of alveolar macrophage depletion and the TLR4-defective C3H/HeJ mouse strain to show that the early NF-κB response that occurs in the lung after RSV infection is dependent upon both alveolar macrophages and TLR4. Early NF-κB activation was “consistently reduced compared with TLR4 competent mice105.” However, NF-κB translocation was observed in the C3H/HeJ mouse at 24 hr p.i., suggesting that TLR4-independent NF-κB inducers are also activated in response to infection. This is not surprising since IL-1β and TNF-α, both NF-κB-inducing cytokines, are induced early in response to RSV infection, and could mediate a second wave of NF-κB. Importantly, other pattern recognition receptors (PRRs) have since been implicated in RSV infection and all activate NF-κB43, 106–108. The role of TLR4 in RSV infection is not without controversy, however, and was challenged by Ehl et al.109. Briefly, the C57BL/10ScCr (TLR4- and IL-12R-deficient) and the C57BL/10ScN (TLR4-deficient) strains were compared after RSV infection. The authors concluded that a transient delay in clearance was attributable to IL-12R, but not TLR4, deficiency. However, these authors stated in their discussion, “The reason for the discrepancy between our study and the two previous studies remains unclear.” In a recent paper, the Finberg laboratory has outlined some factors, i.e., differences in baseline and induced TLR expression in different mouse strains, different doses of RSV used for infection, or status of RSV stocks that present substantial changes in infectivity over time, that could highly affect the study outcome43.
LPS hyporesponsiveness of peripheral blood mononuclear cells (PBMCs) has been identified as a risk factor for intensive care unit hospitalization in infants with RSV bronchiolitis110. Inheritance of two single nucleotide polymorphisms (SNPs) that encode point mutations in the TLR4 ectodomain (Asp299Gly and Thr399Ile) has been associated with decreased LPS responsiveness in vitro and in vivo104, 111. In HEK293T cells that were transiently transfected to express CD14, MD-2, and either wild-type (WT) or mutated TLR4, we showed that the TLR4 variants that express the SNPs respond less robustly than the WT TLR4 to LPS as well as purified RSV F protein, despite equivalent TLR4 expression104. If both SNPs were simultaneously expressed on the same TLR4, the response to both LPS and purified F protein was reduced even further. Subsequently, we identified a highly significant association between RSV infection in a case series of high-risk infants/children with confirmed RSV and inheritance of these two TLR4 SNPs112. DNA from archived nasal lavage samples of participants with documented RSV infection of two early, multi-center clinical trials of antibody-mediated prophylaxis13, 14 were genotyped for the TLR4 ectodomain polymorphisms (Asp299Gly and Thr399Ile). Specifically, 94 of 105 samples from high-risk infants with RSV (89.5%) derived from the original clinical trials of passively administered polyclonal or monoclonal anti-RSV antibodies were heterozygous for the Asp299Gly SNP, in contrast to 742 of 7,092 control healthy individuals (10.5%) derived from data pooled from 25 published studies. Similarly, 92 of 105 (87.6%) of these samples were heterozygous for the Thr399Ile SNP, compared to only 144 of 2,213 healthy controls (6.5%). Thus, inheritance of mutations that confer diminished LPS and F protein responsiveness are found at high frequency in high-risk infants with documented RSV infection. Tal et al.113 also reported that inheritance of these same SNPs correlated with severity of RSV infection in a non-premature cohort.
In addition to TLR4, other TLRs have been implicated in RSV biology. TLR3 has been suggested to participate in cytokine and chemokine production in RSV-infected epithelial cells107, 108. RSV infection of plasmacytoid dendritic cells (pDCs) blocked TLR7/8 signaling107 that are involved in the detection of viral single-stranded RNA. In addition, several laboratories have shown that TLR3 signaling induced by RSV could enhance later responses to the virus107, 114. Boukhvalova et al.,115 recently demonstrated that the pathology associated with RSV infection was increased in cotton rats treated with poly ICLC, a poly IC (polyriboinosinic-polyribocytidylic acid) stabilized with poly-L-lysine carboxymethyl cellulose, and a strong TLR3 agonist. Augmented lung inflammation was accompanied by an early induction of type I and II interferons, TLRs (i.e., TLR1, 2, 3 and 7), and a stronger chemokine (GRO, MCP-1 and MIP-1α) response. In another recent paper, Murawski et al.43 reported that TLR2/6 signaling in leukocytes can activate immunity against RSV by promoting TNF-α, IL-6, MCP-1, and RANTES; this signaling was important for controlling viral replication in vivo. Furthermore, it was demonstrated that RSV-TLR2 interaction promoted neutrophil migration and dendritic cell activation in the lung. In addition to TLRs, other intracellular PRRs, including retinoic acid-inducible gene I (RIG-I) and Nod2, were found to mediate the early antiviral response to RSV by inducing type I IFN 108, 116. Collectively, these data support the existence of multiple PRRs that contribute to the host response to RSV infection and, hence, a more comprehensive characterization of the contribution of each to resistance to RSV will ultimately dictate the composition of a successful vaccine against RSV.
As discussed above, TLR4 signaling is distinguished from TLR2 signaling by the capacity to induce IFN-β via the “MyD88-independent pathway” that contributes to a second wave of transcriptional activity through STAT1 activation99. STAT1−/− mice exhibit severe illness (weight loss, characteristic pulmonary pathology) following RSV infection compared to IFN-γ−/− or WT BALB/c mice, despite similar viral titers and rates of clearance55. These data underscore the important role that type I (α/β) IFNs play in establishing a protective and safe antigen-specific immune response to RSV.
Ancillary TLR4 signaling mitigates pathology and lung cytokine expression in FI-RSV vaccine-enhanced disease
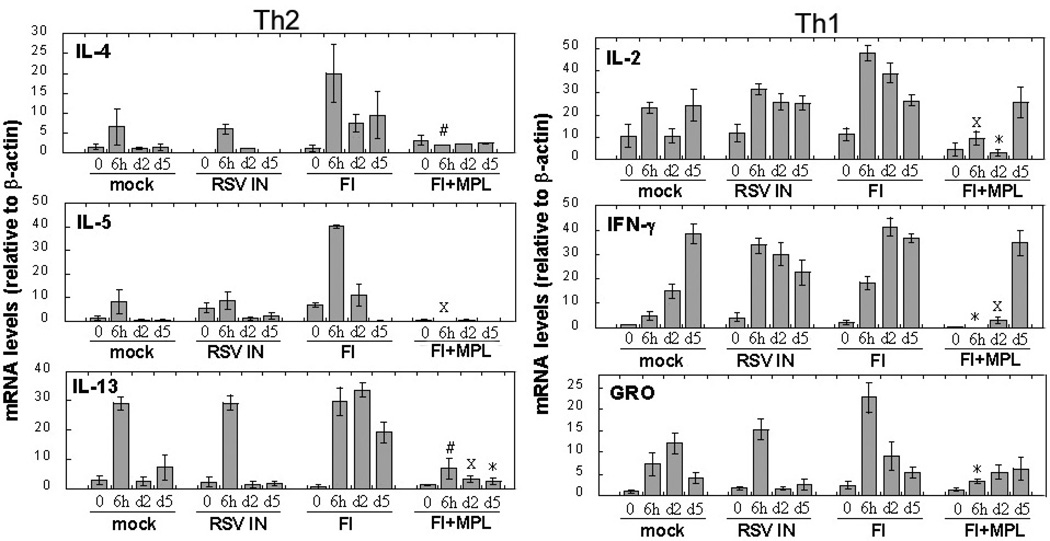
RSV F protein, like LPS, has been reported to be a TLR4 agonist53. We posited that formalin denaturation of F protein would diminish its ability to stimulate TLR4-mediated signaling. Experimental support for this hypothesis was provided by the observation that formaldehyde creates reactive carbonyl groups on RSV proteins that could be associated with vaccine-enhanced disease117. Moghaddam et al. showed that formaldehyde-treated RSV, used as a vaccine, reduced viral replication (1000-fold), but produced enhanced disease in the absence of neutralizing antibody titers. Therefore, we hypothesized that co-administration of a TLR4 agonist with FI-RSV might provide a surrogate signal that would compensate for diminished TLR4 signaling induced by formalin-denatured F protein, and thereby counteract the enhanced pathology and gene expression profile normally induced by FI-RSV vaccination. Since LPS is toxic, even at low doses, due to its ability to elicit a strong proinflammatory response, we utilized monophosphoryl lipid A (MPL), a non-toxic lipid A derivative118 that has been used for over 25 years as an adjuvant and continues to be extensively explored in human vaccine formuations119. Although MPL retains TLR4 agonist activity, it is considerably less potent than LPS and induces in macrophages diminished IL-12 and IFN-γ and increased IL-10 compared to LPS, perhaps accounting, in part, for its decreased toxicity120. Nearly a decade ago, Prince et al.121 immunized cotton rats with FI-RSV vs. FI-RSV + MPL. Upon subsequent RSV infection, cotton rats immunized with the FI-RSV + MPL exhibited greatly mitigated pathology upon subsequent RSV challenge with no protective effect on lung viral replication, despite a slight increase in neutralizing antibody titers. This early observation strongly suggested that protection from the pathologic effects of RSV were dissociable from virus replication (and hence, antibody titers). These findings were confirmed and extended in 2006 by Boukhvalova et al.80. Briefly, cotton rats were immunized twice with FI-RSV (FI) alone or together with MPL (FI+ MPL). Control groups included mock-vaccinated cotton rats (control for primary RSV infection), and cotton rats immunized by live RSV infection (“RSV IN”) as the control for secondary RSV infection. Three weeks after boosting, all animals were challenged with RSV and lungs collected at various times post-infection for analysis of cytokine mRNA expression, histology, and viral load. As initially reported by Prince et al., co-administration of MPL with FI-RSV reversed pathology associated with vaccine-enhanced disease following RSV challenge80. Moreover, cytokine and chemokine gene expression profiles in lung samples revealed several important effects of MPL (Fig. 280). FI-RSV + MPL strongly inhibited expression of Th2 cytokines in response to RSV challenge (Fig. 2; compare “FI” with “FI+ MPL”): peak IL-4, IL-5, and IL-13, but not IL-1080, mRNA were reduced significantly in “FI+ MPL”-vaccinated animals (Fig. 2, Th2). However, expression of all Th1 and other pro-inflammatory cytokine genes (e.g., Fig. 480, Th1) was also significantly reduced early after RSV challenge compared to animals vaccinated with FI-RSV only. MPL similarly dampened expression of all chemokine genes examined (Fig. 480, e.g., GRO). Thus, inclusion of MPL in the FI-RSV vaccine diminishes the host’s capacity to respond pathologically when challenged with RSV by blunting the “cytokine storm” that is normally elicited. These findings strongly suggest that the engagement of TLR4 is critical for mitigating or precluding the pathology normally induced by primary RSV infection following vaccination with FI-RSV with essentially no change in viral replication in the lung (i.e., 3.9; 4.4; and 4.2 log10 pfu RSV/gm lung tissue for FI-RSV, FI-RSV + MPL, and mock vaccination, respectively).
Fig. 2.
MPL blunts the “cytokine storm” associated with FI-RSV vaccine-enhanced disease. Cotton rats were vaccinated i.m. with FI-RSV alone (FI) or in combination with MPL (FI +MPL). Control animals were vaccinated with a mock vaccine (mock) or infected with RSV (RSV IN). After RSV challenge of all groups, animals were sacrificed at the indicated times and lung samples analyzed for expression of Th2 and Th1 cytokine mRNA as described in Fig. 3. Results represent the mean ± S.E.M. for 4 – 6 animals per time point. #, p < 0.05; *, p < 0.01; X, p < 0.001 (FI + MPL vs. FI treatment). Adapted from reference80.
Possible role of increased antibody affinity in blunting of vaccine-enhanced disease
More than four decades have passed since the failed RSV-vaccine trials took place, and still the controversy surrounding the mechanism(s) by which vaccination with FI-RSV predisposes to the development of enhanced pathology upon RSV infection, including the two deaths in those trials, remain a topic of heated debate122, 123. At the time of the failed clinical trials of Lot 100, it was initially thought that FI-RSV pathology was antibody-mediated25. This view went out of favor since studies in cotton rat and subsequent clinical trials demonstrated conclusively that passively administered IgG was highly efficacious in preventing severe RSV pulmonary disease13, 14. A role for antibodies in FI-RSV enhanced disease was revisited when Polack and colleagues demonstrated extensive peribronchiolar deposition of the classical (i.e., antibody-mediated) complement cleavage product C4d in lung sections derived from the two infants that died during the FI-RSV vaccine trials in 1967124. The authors concluded that the pathology seen in FI-RSV-vaccinated children was the result of immune complex deposition containing either high levels of non-neutralizing antibodies generated by the poorly immunogenic FI-RSV vaccine, or abundant low avidity antibodies derived from B cells that underwent “altered maturation.” More recently, Delgado et al. reported that immunization of mice with FI-RSV or UV-inactivated RSV resulted in enhanced airway disease and lung pathology upon RSV challenge and correlated this with the production of low-avidity anti-RSV antibodies with a decreased IgG2a/IgG1 ratio122. Consistent with our earlier publications that the TLR4 agonist, MPL, mitigated enhanced disease induced by RSV infection of cotton rats vaccinated with FI-RSV80, 121, Delgado et al. also reported that vaccinating mice with UV-RSV plus the potent TLR4 agonist, LPS, reduced enhanced RSV disease122. They concluded that inclusion of a TLR4 agonist with the UV-RSV blunted vaccine-enhanced disease by inducing B cell affinity maturation leading to increased antibody avidity. However, their conclusions have been recently challenged by Shaw et al.123 who pointed out that the data presented by Delgado et al., “do not explain why an RSV vaccine that fails to block infection actually enhances subsequent disease.” (i.e., why is the pathology worsened over that seen in primary RSV infection?). Experiments using sensitive animal models vaccinated with the original Lot 100 vaccine and subsequently administered passively transferred antibodies of differing affinities will be required to test directly the correlation between antibody avidity and isotype, viral titers, and more importantly, protection from vaccine-enhanced RSV-induced pathology.
Possible role of alternatively activated macrophages in preventing RSV-induced pathology in primary and secondary infection
“Classically activated” macrophages (CA-Mϕ) differentiate in response to inflammatory stimuli, such as IFN-γ, in combination with TLR activation by microbial stimuli, such as Gram negative LPS. CA-Mϕ kill intracellular pathogens and secrete inflammatory cytokines that amplify Th1 immune responses. CA-Mϕ are also associated with the pathology seen in many inflammatory diseases and produce iNOS, the enzyme that generates nitric oxide (NO) that can damage cells. “Alternatively activated” macrophages (AA-Mϕ) differentiate in response to the Th2 cytokines, IL-4 and IL-13, and are functionally and biochemically distinct from CA-Mϕ. AA-Mϕ produce arginase-1 that competes with iNOS for arginine to produce L-ornithine and urea, rather than NO125 as well as chitinase enzymes that have been implicated in tissue repair126. Although much work on the response to RSV has focused on whether a “Th1” or “Th2” adaptive immune response is induced, it is surprising that essentially no attention has been paid to RSV-induced differentiation of CA-Mϕ vs. AA-M ϕ, since their cytokine profiles are so similar to that of Th1 and Th2 cells, respectively.
A recent report by Shirey et al.58 provides new insights into a highly novel mechanism by which RSV-induced lung pathology may be controlled. Specifically, RSV infection of murine lung and peritoneal macrophages, as well as a macrophage cell line, resulted in IL-4 and IL-13 production, IL-4Rα/STAT6-dependent AA-Mϕ differentiation, and led to significantly enhanced inflammation in lungs of IL-4Rα−/− mice. IL-4Rα is required for both IL-4- and IL-13-mediated signaling127. Adoptive transfer of highly purified WT macrophages to IL-4Rα−/− mice restored RSV-inducible AA-Mϕ phenotype and diminished lung pathology. RSV-infected TLR4−/− and IFN-β−/− macrophages and TLR4−/− and IFN-β−/− mice also failed to express AA-Mϕ markers, but exhibited sustained proinflammatory cytokine production (e.g., IL-12) in vitro and in vivo and epithelial damage in vivo. TLR4 signaling was found to be required for RSV-induced PPARγ expression, a DNA-binding protein that induces AA-Mϕ genes, while IFN-β regulates IL-4, IL-13, IL-4Rα, and IL-10 expression in response to RSV. Thus, TLR4- and IFN-β̃-mediated signals contribute to the amelioration of RSV-induced pathology. It is tempting to speculate that the remarkable association between RSV susceptibility and the TLR4 SNPs that we previously reported might reflect a diminished responsiveness to RSV F protein or endogenous TLR4 agonists generated during RSV infection, thereby leading to a decreased capacity for development of tissue reparative AA-Mϕ.
Secondary infection of cotton rats with RSV results in faster resolution of inflammation and no detectable viral titers60. When cotton rats were infected once, IL-4 and IL-13 mRNA were detected in the lungs during primary infection, but these genes, as well as the A-AMϕ marker, arginase-1 mRNA, were more strongly activated upon re-infection 60 days later58. This observation suggests that AA-Mϕ development during primary infection facilitates a less robust secondary RSV infection.
Cyclooxygenase 2 (COX-2) was previously shown in cotton rats to contribute to RSV-mediated pathology, as evidenced by the fact that COX-2- or prostaglandin E-specific inhibitors blocked RSV-induced pathology128. Treatment of RSV-infected cotton rats with a COX-2-specific inhibitor also increased expression of lung AA-Mϕ. Future studies using FI-RSV-vaccinated mice and cotton rats will be required to determine if manipulation of the macrophage activation state in vivo will also protect against vaccine-enhanced disease. Obviously, treating severely ill patients with agents or vaccines that favor development of AA-Mϕ might be expected to predispose the patient to airway hyperreactivity; however, given that our current therapeutic interventions often fail to save patients with severe RSV disease, the critical balance of mitigating severe RSV disease with managing asthma in later life may have to be accepted until a safe and efficacious vaccine can be developed.
Concluding remarks
The genetic and immunological components of the host that led to the disastrous results of vaccine trials of FI-RSV remain an enigma. The delineation of the mechanisms underlying vaccine-enhanced disease will require robust animal models that closely mimic the key components of the disease. The role that antibodies and cell-mediated immune responses play in the overall pathology of FI-RSV and the genetics of RSV sensitivity will continue to be grounds for investigation. However, the interaction of the F protein of RSV and TLR4 has emerged as an important component in the multifactorial host response to RSV infection that should be considered when developing vaccine to achieve safe, long-lasting protection against RSV.
Acknowledgment
This work was supported by National Institute of Health, National Institute of Infectious Diseases Grants AI-057575 (JCGB) and AI18797 (SNV).
References
- 1.Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–S117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 2.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children 1980–1996. Jama. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 3.Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979#x02013;1997. J Infect Dis. 2001;183:16–22. doi: 10.1086/317655. [DOI] [PubMed] [Google Scholar]
- 4.Schickli JH, Dubovsky F, Tang RS. Challenges in developing a pediatric RSV vaccine. Hum Vaccin. 2009;5:582–591. doi: 10.4161/hv.9131. [DOI] [PubMed] [Google Scholar]
- 5.Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179:25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 6.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 7.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington RD, Hooton TM, Hackman RC, et al. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992;165:987–993. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- 9.Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27–30. doi: 10.1016/s0002-9343(97)00007-7. discussion 42-3. [DOI] [PubMed] [Google Scholar]
- 10.Henderson FW, Collier AM, Clyde WA, Jr, Denny FW. Respiratory syncytial virus infections, reinfections and immunity A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 11.Collins PL, Crowe JE. Respiratory syncytial virus and metapneumovirus. In: Knipe DM, Howley PM, editors. Field virology. Vol. 2. Philadelphia: Lippincott-Williams & Wilkins; 2007. pp. 1601–1646. [Google Scholar]
- 12.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505. [PubMed] [Google Scholar]
- 13.The Prevent Study Group. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–99. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 14.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 15.American Association of Pediatrics (AAP) Summary of Recommendations of the AAP Committee on Infectious Diseases. AAP News. 2002;21:232–233. [Google Scholar]
- 16.Collins PL, Huang YT, Wertz GW. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1984;81:7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karron RA, Buonagurio DA, Georgiu AF, et al. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc Natl Acad Sci U S A. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olmsted RA, Elango N, Prince GA, et al. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci U S A. 1986;83:7462–7466. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pemberton RM, Cannon MJ, Openshaw PJ, Ball LA, Wertz GW, Askonas BA. Cytotoxic T cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J Gen Virol. 1987;68(Pt 8):2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- 20.Johnson S, Oliver C, Prince GA, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infet Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 21.Beeler JA, van Wyke Coelingh K. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J Virol. 1989;63:2941–2950. doi: 10.1128/jvi.63.7.2941-2950.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 23.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 24.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 25.Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 26.McIntosh K, Chanock RM. Respiratory syncytial virus, Chapter 38. In: Fields BN, editor. Virology. 1990. pp. 1045–1072. [Google Scholar]
- 27.Prince GA, Horswood RL, Camargo E, Koenig D, Chanock RM. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983;42:81–87. doi: 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prince GA, Horswood RL, Chanock RM. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55:517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemming VG, Rodriguez W, Kim HW, et al. Intravenous immunoglobulin treatment of respiratory syncytial virus infections in infants and young children. Antimicrob Agents Chemother. 1987;31:1882–1886. doi: 10.1128/aac.31.12.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright PF, Karron RA, Belshe RB, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 31.Karron RA, Wright PF, Belshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 32.Groothuis JR, King SJ, Hogerman DA, Paradiso PR, Simoes EA. Safety and immunogenicity of a purified F protein respiratory syncytial virus (PFP-2) vaccine in seropositive children with bronchopulmonary dysplasia. J Infect Dis. 1998;177:467–469. doi: 10.1086/517377. [DOI] [PubMed] [Google Scholar]
- 33.Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol. 2003;77:13156–13160. doi: 10.1128/JVI.77.24.13156-13160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piedra PA, Cron SG, Jewell A, et al. Immunogenicity of a new purified fusion protein vaccine to respiratory syncytial virus: a multi-center trial in children with cystic fibrosis. Vaccine. 2003;21:2448–2460. doi: 10.1016/s0264-410x(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 35.Prince GA, Capiau C, Deschamps M, et al. Efficacy and safety studies of a recombinant chimeric respiratory syncytial virus FG glycoprotein vaccine in cotton rats. J Virol. 2000;74:10287–10292. doi: 10.1128/jvi.74.22.10287-10292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du RP, Jackson GE, Wyde PR, et al. A prototype recombinant vaccine against respiratory syncytial virus and parainfluenza virus type 3. Biotechnology (N Y) 1994;12:813–818. doi: 10.1038/nbt0894-813. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Sambhara S, Li CX, et al. Plasmid DNA encoding the respiratory syncytial virus G protein is a promising vaccine candidate. Virology. 2000;269:54–65. doi: 10.1006/viro.2000.0186. [DOI] [PubMed] [Google Scholar]
- 38.Bembridge GP, Rodriguez N, Garcia-Beato R, Nicolson C, Melero JA, Taylor G. DNA encoding the attachment (G) or fusion (F) protein of respiratory syncytial virus induces protection in the absence of pulmonary inflammation. J Gen Virol. 2000;81:2519–2523. doi: 10.1099/0022-1317-81-10-2519. [DOI] [PubMed] [Google Scholar]
- 39.Park EK, Soh BY, Jang YS, Park JH, Chung GH. Immune induction and modulation in mice following immunization with DNA encoding F protein of respiratory syncytial virus. Mol Cells. 2001;12:50–56. [PubMed] [Google Scholar]
- 40.Vaughan K, Rhodes GH, Gershwin LJ. DNA immunization against respiratory syncytial virus (RSV) in infant rhesus monkeys. Vaccine. 2005;23:2928–2942. doi: 10.1016/j.vaccine.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 41.Wu H, Dennis VA, Pillai SR, Singh SR. RSV fusion (F) protein DNA vaccine provides partial protection against viral infection. Virus Res. 2009;145:39–47. doi: 10.1016/j.virusres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dagouassat N, Robillard V, Haeuw JF, et al. A novel bipolar mode of attachment to aluminium-containing adjuvants by BBG2Na, a recombinant subunit hRSV vaccine. Vaccine. 2001;19:4143–4152. doi: 10.1016/s0264-410x(01)00168-2. [DOI] [PubMed] [Google Scholar]
- 43.Murawski MR, Bowen GN, Cerny AM, et al. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Waal L, Power UF, Yuksel S, et al. Evaluation of BBG2Na in infant macaques: specific immune responses after vaccination and RSV challenge. Vaccine. 2004;22:915–922. doi: 10.1016/j.vaccine.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Luxon BA, Casola A, Garofalo RP, Jamaluddin M, Brasier AR. Expression of Respiratory Syncytial Virus-Induced Chemokine Gene Networks in Lower Airway Epithelial Cells Revealed by cDNA Microarrays. J Virol. 2001;75:9044–9058. doi: 10.1128/JVI.75.19.9044-9058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dreizin RS, Vyshnevetskaia LO, Bagdamian EE, Iankevich OD, Tarasova LB. Experimental RS virus infection of cotton rats. A viral and immunofluorescent study. Vopr Virusol. 1971;16:670–676. [PubMed] [Google Scholar]
- 47.Prince GA, Horswood RL, Berndt J, Suffin SC, Chanock RM. Respiratory syncytial virus infection in inbred mice. Infect Immun. 1979;26:764–766. doi: 10.1128/iai.26.2.764-766.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prince GA, Jenson AB, Horswood RL, Camargo E, Chanock RM. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978;93:771–791. [PMC free article] [PubMed] [Google Scholar]
- 49.Prince GA, Hemming VG, Horswood RL, Chanock RM. Immunoprophilaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 1985;3:193–206. doi: 10.1016/0168-1702(85)90045-0. [DOI] [PubMed] [Google Scholar]
- 50.Meissner HC, Welliver RC, Chartrand SA, et al. Immunoprophylaxis with palivizumab, a humanized respiratory syncytial virus monoclonal antibody, for prevention of respiratory syncytial virus infection in high risk infants: a consensus opinion. Pediatr Infect Dis J. 1999;18:223–231. doi: 10.1097/00006454-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Prince GA, Jenson AB, Hemming VG, et al. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. J Virol. 1986;57:721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussell T, Georgiou A, Sparer TE, Matthews S, Pala P, Openshaw PJ. Host genetic determinants of vaccine-induced eosinophilia during respiratory syncytial virus infection. J Immunol. 1998;161:6215–6222. [PubMed] [Google Scholar]
- 53.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 54.van Schaik SM, Obot N, Enhorning G, et al. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. J Med Virol. 2000;62:257–266. doi: 10.1002/1096-9071(200010)62:2<257::aid-jmv19>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 55.Durbin JE, Johnson TR, Durbin RK, et al. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol. 2002;168:2944–2952. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- 56.Johnson TR, Mertz SE, Gitiban N, et al. Role for innate IFNs in determining respiratory syncytial virus immunopathology. J Immunol. 2005;174:7234–7241. doi: 10.4049/jimmunol.174.11.7234. [DOI] [PubMed] [Google Scholar]
- 57.Zhou W, Hashimoto K, Moore ML, et al. IL-13 is associated with reduced illness and replication in primary respiratory syncytial virus infection in the mouse. Microbes Infect. 2006;8:2880–2889. doi: 10.1016/j.micinf.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirey KA, Pletneva L, Puche A, et al. Control of RSV-induced lung injury by alternatively activated Macrophages is IL-4Rα-, TLR4-, and IFN-β-dependent. Mucosal Imm. doi: 10.1038/mi.2010.6. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Durbin JE, Durbin RK. Respiratory syncytial virus-induced immunoprotection and immunopathology. Viral Immunol. 2004;17:370–380. doi: 10.1089/vim.2004.17.370. [DOI] [PubMed] [Google Scholar]
- 60.Prince GA, Prieels JP, Slaoui M, Porter DD. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus) Lab Invest. 1999;79:1385–1392. [PubMed] [Google Scholar]
- 61.Prince GA, Curtis SJ, Yim KC, Porter DD. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol. 2001;82:2881–2888. doi: 10.1099/0022-1317-82-12-2881. [DOI] [PubMed] [Google Scholar]
- 62.Johnson TR, Johnson JE, Roberts SR, Wertz GW, Parker RA, Graham BS. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J Virol. 1998;72:2871–2880. doi: 10.1128/jvi.72.4.2871-2880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tebbey PW, Hagen M, Hancock GE. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J Exp Med. 1998;188:1967–1972. doi: 10.1084/jem.188.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–107. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- 65.Kakuk TJ, Soike K, Brideau RJ, et al. A human respiratory syncytial virus (RSV) primate model of enhanced pulmonary pathology induced with a formalin-inactivated RSV vaccine but not a recombinant FG subunit vaccine. J Infect Dis. 1993;167:553–561. doi: 10.1093/infdis/167.3.553. [DOI] [PubMed] [Google Scholar]
- 66.Gershwin LJ, Schelegle ES, Gunther RA, et al. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine. 1998;16:1225–1236. doi: 10.1016/s0264-410x(98)80123-0. [DOI] [PubMed] [Google Scholar]
- 67.Connors M, Kulkarni AB, Collins PL, et al. Resistance to respiratory syncytial virus (RSV) challenge induced by infection with a vaccinia virus recombinant expressing the RSV M2 protein (Vac-M2) is mediated by CD8+ T cells, while that induced by Vac-F or Vac-G recombinants is mediated by antibodies. J Virol. 1992;66:1277–1281. doi: 10.1128/jvi.66.2.1277-1281.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 69.Sparer TE, Matthews S, Hussell T, et al. Eliminating a region of respiratory syncytial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J Exp Med. 1998;187:1921–1926. doi: 10.1084/jem.187.11.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roman M, Calhoun WJ, Hinton KL, et al. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med. 1997;156:190–195. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 71.Bont L, Heijnen CJ, Kavelaars A, et al. Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis. 2001;184:355–358. doi: 10.1086/322035. [DOI] [PubMed] [Google Scholar]
- 72.Chen ZM, Mao JH, Du LZ, Tang YM. Association of cytokine responses with disease severity in infants with respiratory syncytial virus infection. Acta Paediatr. 2002;91:914–922. doi: 10.1080/080352502760272588. [DOI] [PubMed] [Google Scholar]
- 73.Tripp RA, Moore D, Barskey At, et al. Peripheral blood mononuclear cells from infants hospitalized because of respiratory syncytial virus infection express T helper-1 and T helper-2 cytokines and CC chemokine messenger RNA. J Infect Dis. 2002;185:1388–1394. doi: 10.1086/340505. [DOI] [PubMed] [Google Scholar]
- 74.Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168:633–639. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 75.Semple MG, Dankert HM, Ebrahimi B, et al. Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. PLoS One. 2007;2:e1038. doi: 10.1371/journal.pone.0001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Welliver TP, Garofalo RP, Hosakote Y, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Byeon JH, Lee JC, Choi IS, Yoo Y, Park SH, Choung JT. Comparison of cytokine responses in nasopharyngeal aspirates from children with viral lower respiratory tract infections. Acta Paediatr. 2009;98:725–730. doi: 10.1111/j.1651-2227.2008.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis. 2001;184:393–399. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]
- 79.Brandenburg AH, Kleinjan A, van Het Land B, et al. Type 1-like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J Med Virol. 2000;62:267–277. [PubMed] [Google Scholar]
- 80.Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JC. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine. 2006;24:5027–5035. doi: 10.1016/j.vaccine.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 81.Blanco JC, Boukhvalova M, Pletneva L, Prince GA, Vogel SN. Re-thinking Respiratory Syncytial Virus vaccines Recent Res. Devel. Experimental Med. 2004;1:75–94. [Google Scholar]
- 82.Zhou Y, McLane M, Levitt RC. Th2 cytokines and asthma. Interleukin-9 as a therapeutic target for asthma. Respir Res. 2001;2:80–84. doi: 10.1186/rr42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dodd JS, Lum E, Goulding J, Muir R, Van Snick J, Openshaw PJ. IL-9 regulates pathology during primary and memory responses to respiratory syncytial virus infection. J Immunol. 2009;183:7006–7013. doi: 10.4049/jimmunol.0900085. [DOI] [PubMed] [Google Scholar]
- 84.Yim KC, Cragin RP, Boukhvalova MS, et al. Human metapneumovirus: Enhanced pulmonary disease in cotton rats immunized with formalin-inactivated virus vaccine and challenged. Vaccine. 2007;25:5034–5040. doi: 10.1016/j.vaccine.2007.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haziot A, Ferrero E, Kontgen F, et al. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 86.Perera PY, Vogel SN, Detore GR, Haziot A, Goyert SM. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J Immunol. 1997;158:4422–4429. [PubMed] [Google Scholar]
- 87.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 88.Poltorak A, Smirnova I, He X, et al. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis. 1998;24:340–355. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 89.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 90.Qureshi ST, Lariviere L, Leveque G, et al. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 92.Perera PY, Mayadas TN, Takeuchi O, et al. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol. 2001;166:574–581. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 93.Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–38051. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 95.Gioannini TL, Teghanemt A, Zhang D, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci U S A. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim HM, Park BS, Kim JI, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906–917. doi: 10.1016/j.cell.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 98.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 99.Toshchakov V, Jones BW, Perera PY, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 100.Gao JJ, Diesl V, Wittmann T, et al. Regulation of gene expression in mouse macrophages stimulated with bacterial CpG-DNA and lipopolysaccharide. J Leukoc Biol. 2002;72:1234–1245. [PubMed] [Google Scholar]
- 101.Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol. 2001;69:598–604. [PubMed] [Google Scholar]
- 102.Vogel SN, Fitzgerald KA, Fenton MJ. TLRs: differential adapter utilization by toll-like receptors mediates TLR-specific patterns of gene expression. Mol Interv. 2003;3:466–477. doi: 10.1124/mi.3.8.466. [DOI] [PubMed] [Google Scholar]
- 103.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rallabhandi P, Bell J, Boukhvalova MS, et al. Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol. 2006;177:322–332. doi: 10.4049/jimmunol.177.1.322. [DOI] [PubMed] [Google Scholar]
- 105.Haeberle HA, Takizawa R, Casola A, et al. Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and toll-like receptor 4-dependent pathways. J Infect Dis. 2002;186:1199–1206. doi: 10.1086/344644. [DOI] [PubMed] [Google Scholar]
- 106.Schlender J, Hornung V, Finke S, et al. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ehl S, Bischoff R, Ostler T, et al. The role of Toll-like receptor 4 versus interleukin-12 in immunity to respiratory syncytial virus. Eur J Immunol. 2004;34:1146–1153. doi: 10.1002/eji.200324449. [DOI] [PubMed] [Google Scholar]
- 110.Mandelberg A, Tal G, Naugolny L, et al. Lipopolysaccharide hyporesponsiveness as a risk factor for intensive care unit hospitalization in infants with respiratory syncitial virus bronchiolitis. Clin Exp Immunol. 2006;144:48–52. doi: 10.1111/j.1365-2249.2006.03030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 112.Awomoyi AA, Rallabhandi P, Pollin TI, et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007;179:3171–3177. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- 113.Tal G, Mandelberg A, Dalal I, et al. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. J Infect Dis. 2004;189:2057–2063. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 114.Groskreutz DJ, Monick MM, Yarovinsky TO, et al. Respiratory syncytial virus decreases p53 protein to prolong survival of airway epithelial cells. J Immunol. 2007;179:2741–2747. doi: 10.4049/jimmunol.179.5.2741. [DOI] [PubMed] [Google Scholar]
- 115.Boukhvalova MS, Sotomayor TB, Point RC, Pletneva LM, Prince GA, Blanco JC. Activation of Interferon Response Through Toll-Like Receptor 3 Impacts Viral Pathogenesis and Pulmonary Toll-Like Receptor Expression During Respiratory Syncytial Virus and Influenza Infections in the Cotton Rat Sigmodon hispidus Model. J Interferon Cytokine Res. 2009 doi: 10.1089/jir.2009.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sabbah A, Chang TH, Harnack R, et al. Activation of innate immune antiviral responses by Nod2. Nat Immun. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moghaddam A, Olszewska W, Wang B, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12:905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 118.Qureshi N, Takayama K, Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982;257:11808–11815. [PubMed] [Google Scholar]
- 119.Alderson MR, McGowan P, Baldridge JR, Probst P. TLR4 agonists as immunomodulatory agents. J Endotoxin Res. 2006;12:313–319. doi: 10.1179/096805106X118753. [DOI] [PubMed] [Google Scholar]
- 120.Salkowski CA, Detore GR, Vogel SN. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in murine macrophages. Infect Immun. 1997;65:3239–3247. doi: 10.1128/iai.65.8.3239-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Prince GA, Denamur F, Deschamps M, et al. Monophosphoryl lipid A adjuvant reverses a principal histologic parameter of formalin-inactivated respiratory syncytial virus vaccine-induced disease. Vaccine. 2001;19:2048–2054. doi: 10.1016/s0264-410x(00)00417-5. [DOI] [PubMed] [Google Scholar]
- 122.Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shaw CA, Otten G, Wack A, et al. Antibody affinity maturation and respiratory syncytial virus disease. Nat Med. 2009;15:725. doi: 10.1038/nm0709-725a. author reply 725-6. [DOI] [PubMed] [Google Scholar]
- 124.Polack FP, Teng MN, Collins PL, et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J Exp Med. 2002;196:859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 126.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 127.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connection maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 128.Richardson JY, Ottolini MG, Pletneva L, et al. Respiratory syncytial virus (RSV) infection induces cyclooxygenase 2: a potential target for RSV therapy. J Immunol. 2005;174:4356–4364. doi: 10.4049/jimmunol.174.7.4356. [DOI] [PubMed] [Google Scholar]