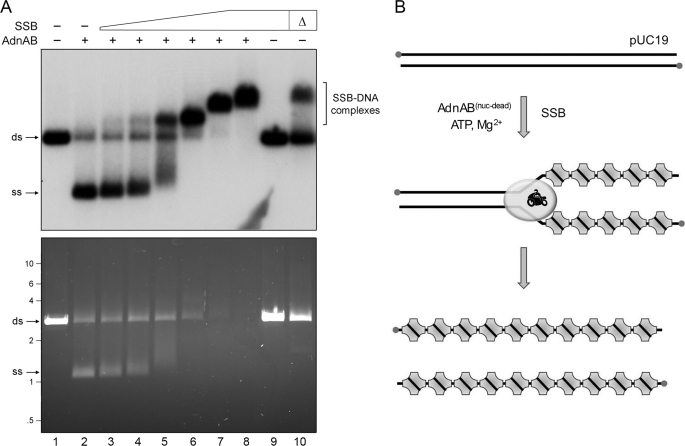
FIGURE 3.
SSB captures the strands unwound by the AdnAB motor. A, reaction mixtures (10 μl) containing 20 mm Tris-HCl, pH 8.0, 1 mm DTT, 2 mm MgCl2, 1 mm ATP, 200 ng of 5′ 32P-labeled pUC19 DNA (BamHI-digested; 230 fmol of DSB ends), 1.06 pmol of nuclease-dead AdnAB (where indicated by +), and increasing amounts of SSB, either 3.6 (lane 3), 7.2 (lane 4), 14.4 (lane 5), 28.8 (lane 6), 57.5 (lane 7), or 115 (lanes 8 and 9) pmol of SSB monomer, were incubated for 10 min at 37 °C. The reactions were quenched by adjusting the mixtures to 50 mm EDTA, 15% glycerol, 0.125% Orange-G dye. The mixture in lane 10, lacking AdnAB, was heated for 5 min at 95 °C (Δ) and then chilled on ice prior to adding SSB (115 pmol). The reaction products were analyzed by electrophoresis through a 0.8% native agarose gel in 50 mm Tris acetate, 2.5 mm EDTA. After visualizing the DNA by staining with ethidium bromide (bottom panel), the gel was dried under vacuum on DE81 paper, and radiolabeled DNA was visualized by autoradiography of the dried gel (top panel). B, reaction scheme is illustrated, whereby SSB tetramers bind to the single-stranded DNA formed in the wake of the advancing AdnAB motor to yield SSB·ssDNA complexes as end products. ds, double strand; ss, single strand.