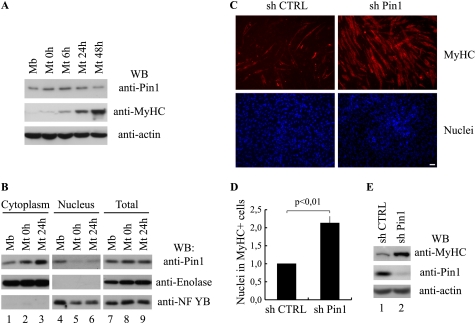
FIGURE 1.
Knockdown of Pin1 promotes skeletal muscle differentiation. A, Western blot (WB) analysis of Pin1, MyHC, and actin protein levels during myogenic differentiation. Total protein extracts from proliferating (Mb) or differentiating (Mt 0h, 6h, 24h, and 48h) C2C7 muscle cells were analyzed by Western blot using the antibodies against Pin1, MyHC, and actin. B, subcellular localization of Pin1 during skeletal muscle differentiation. Cytoplasmic (lanes 1–3), nuclear (lanes 4–6), and total (lanes 7–9) protein extracts from proliferating (Mb) or differentiating (Mt 0h and Mt 24h) C2C7 muscle cells were analyzed by Western blot using the antibody against Pin1; furthermore, we checked the quality of the subcellular protein extracts with antibodies against the glycolytic enzyme enolase and the nuclear transcription factor NF-YB. C, C2C7 myoblasts were infected with lentiviruses encoding short hairpin RNAs (a scramble control sequence (sh CTRL) or a Pin1 silencing sequence (sh Pin1), respectively) and then induced to differentiate. After 48 h from serum withdrawal, cells were fixed and subjected to immunostaining using the anti-MyHC antibody (red stain, upper panels), cell nuclei were stained by Hoechst (lower panels). Bar, 50 μm. D, the proportion of nuclei in differentiated cells is reported as ratio between the nuclei incorporated in MyHC-positive cells and total nuclei. The number obtained in Pin1-depleted cells (sh Pin1) is expressed relative to the number evaluated in cells infected with the scramble control vector (sh CTRL) taken as 1. The data are presented in the histogram and represent the mean ± S.D. (error bars) of three independent experiments (p < 0,01). E, Western blot analysis performed on protein extracts of infected C2C7 cells with antibodies against MyHC, Pin1, and actin.