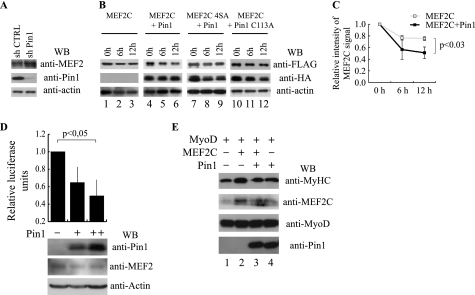
FIGURE 6.
Pin1 controls MEF2C stability and activity. A, Western blot analysis of MEF2, Pin1, and actin protein levels performed on protein extracts of C2C7 myoblasts infected with lentiviruses encoding short hairpin RNAs, a scramble control sequence (shCTRL) or a Pin1 silencing sequence (sh Pin1), respectively. B, protein stability assay. FLAG MEF2C wild type or mutated on the four phosphoacceptor sites (4SA) were overexpressed in COS1 cells either alone or with HA-tagged Pin1 as indicated. After 36 h, protein synthesis was blocked with cycloheximide, and the amount of FLAG MEF2C and HA Pin1 remaining at different times was checked by Western blot. Protein loading was controlled by anti-actin staining. C, the intensity of the wild type MEF2C bands of the experiment in B was quantified by densitometric scanning. The chart reports the average of the relative intensity of MEF2C signals for three independent experiments (p < 0.03). D, effect of Pin1 on the transcriptional activity of endogenous MEF2 proteins. C2C7 cells were transfected with 1 μg (+) or 3 μg (++) of the HA-Pin1 expression plasmid together with the pGL3(desMEF2)3 luciferase and the pRSVβ-gal reporter plasmids. Twenty-four hours after transfection, cells were shifted to low serum medium and kept in culture for an additional 24 h. Luciferase activity was determined and normalized to the β-gal activity. Statistical analysis was performed on data obtained from four independent experiments. The normalized luciferase activities are expressed relative to the sample transfected with the empty expression vector. The amounts of transfected HA-Pin1, endogenous MEF2 proteins, and actin were monitored by Western blot analysis using the anti-Pin1, anti-MEF2, and anti-actin antibodies, respectively. E, Pin1 blocks cooperative activation of myogenic conversion by MEF2C and MyoD. C3H10T1/2 fibroblasts were transiently transfected with the indicated plasmids. After 48 h in growth medium, cells were induced to differentiate in differentiation medium for 6 days and then lysed. Whole cell extracts were subjected to immunoblotting for MyHC, MEF2C, MyoD, and Pin1. Error bars, S.D.