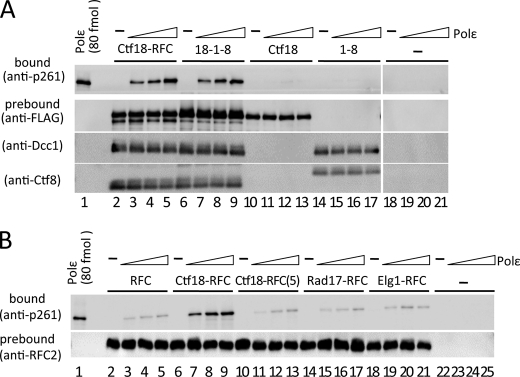
FIGURE 2.
Co-immunoprecipitation of pol ϵ with loader complexes. A, approximately 0.7 pmol of Ctf18-RFC (lanes 2–5), 18-1-8 (lanes 6–9), Ctf18 (lanes 10–13), and 1-8 (with FLAG-Ctf8, lanes 14–17) prebound to anti-FLAG beads was co-immunoprecipitated with 0 (−), 0.2, 0.4, and 0.6 pmol (triangles) of pol ϵ in 10-μl reaction mixtures. Lanes 18–21 are the negative control without Ctf18 complexes. Input pol ϵ (80 fmol) was applied as the loading standard (lane 1). One-fifth of each precipitate was analyzed by immunoblotting with an anti-p261 monoclonal antibody (top panels). Prebound proteins (FLAG-Ctf18, Dcc1, and Ctf8 or FLAG-Ctf8) were compared by immunoblotting. B, 0.7 pmol of RFC (lanes 2–5), Ctf18-RFC (lanes 6–9), Ctf18-RFC(5) (lanes 10–13), Rad17-RFC (lanes 14–17), and Elg1-RFC (lanes 18–21) prebound to anti-FLAG beads was incubated with increasing amounts of pol ϵ and analyzed by immunoblotting as in A. Lanes 22–25 are the negative control without loaders. Immunoblotting of RFC2 shows the uniformity of the bound loader proteins.