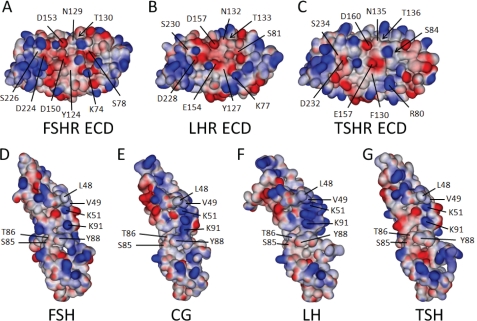
FIGURE 7.
Structures and models of the three GpHR ECDs and bound cognate hormones. Shown are the electrostatic surfaces and relevant amino acid residues (each indicated with a line; those buried with an arrow) studied herein, displayed using an orientation showing the hormone-binding interface of the FSHR (A), LHR (B), and TSHR (C). Also shown are the electrostatic surfaces of the three cognate hormones, FSH (D), CG (E), LH (F), and TSH (G), with the interacting residues highlighted. The structures of the FSHR ECD and FSH are taken from the crystal structure of the complex (30) and that for the TSHR ECD is from the M22·TSHR ECD complex (32). The predicted structures of bound LHR, CG, LH, and TSH were modeled from the published structures of free human CG (55, 56), free FSH (57), and the FSH·FSHR complex (30) and have been separated to enhance visibility of the interacting surfaces. As discussed in the text, CG has a more positive charge localization than FSH and TSH in a region predicted to be part of the binding complex. E, this localization is defined in large part by the close proximity of α-subunit residues Lys51 and Lys91 and β-subunit residue Arg95. Protorp outputs for the two forms of the FSH·FSHR ECD complex (30) are given in supplemental Table 2, along with those for our models of the CG·LHR ECD and TSH·TSHR ECD complexes. The supplemental Table 3 lists the root mean square errors and Ramachandran values for the hormones and receptors, indicating that the models generated provide good structures.