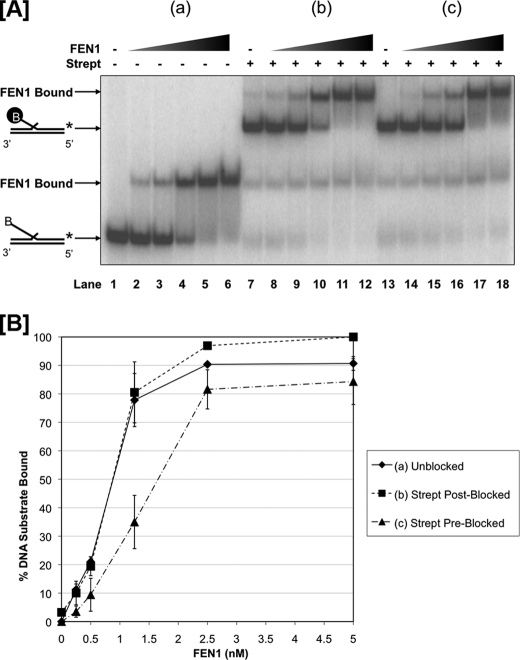
FIGURE 1.
FEN1 binds similarly to unblocked and blocked 53 nt flap substrates independent of threading. FEN1 binding was measured by EMSA as described under “Experimental Procedures.” Reactions were initiated by incubating increasing concentrations of FEN1 (0.25, 0.5, 1.25, 2.5, and 5 nm) with the experimental substrate for 15 min. A, shows FEN1 bound to a 53 nt 5′ biotinylated flap substrate (U1:T1:D1.53B) when (a) no streptavidin was added to the reaction (lanes 1–6), (b) streptavidin was added 10 min after reaction initiation (lanes 7–12), and (c) streptavidin was added 10 min before reaction initiation (lanes 13–18). Lane 1 shows the substrate alone control. Lanes 7 and 13 show the streptavidin-bound substrate controls. The positions of the substrate alone and FEN1-substrate complex are indicated to the left of the figure. A “B” in the oligonucleotide sequence indicates the location of the 5′ biotin, and the black-circled “B” represents streptavidin-bound biotin. B shows the graphical quantitation of A based on at least three independent EMSA results.