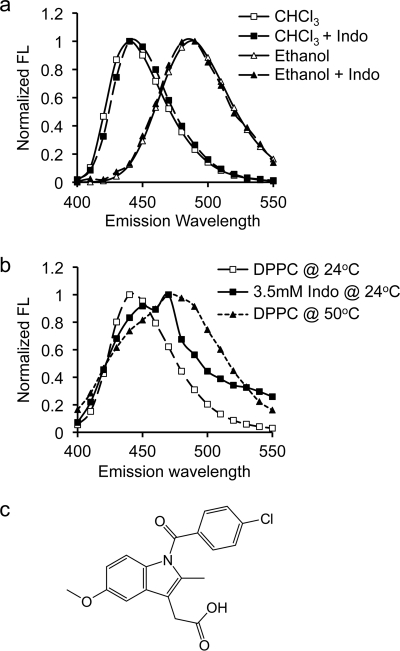
FIGURE 1.
Emission spectral shift of Laurdan in organic solvents and phospholipid bilayers. a, emission spectral shift of Laurdan depends on the polarity of the surrounding environment. In non-polar chloroform (diamonds), Laurdan emission spectrum (excited at 340 nm) displays a peak at 440 nm. Exposure to polar ethanol (circles) shifts the emission spectrum to the right to 490 nm. The presence of Indo has no effect on emission spectra of Laurdan in either solvent, confirming no direct interaction between indomethacin molecules and the probe. b, in a phospholipid membrane, the emission spectral shift of Laurdan depends on the membrane phase behavior. The spectral peak of Laurdan in gel phase DPPC (at 24 °C) occurs at 440 nm. Increasing the temperature to 50 °C shifts the emission peak to 490 nm, indicating a transition to liquid phase. Addition of 3.5 mm indomethacin at 24 °C moves the Laurdan emission peak to a position between gel phase and liquid phase of DPPC. Two peaks are found: at ∼450 nm and ∼470 nm. c, chemical structure of indomethacin.