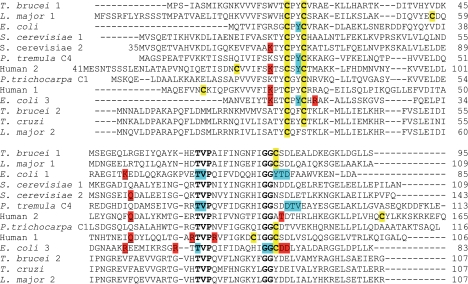
FIGURE 1.
Comparison of T. brucei Grx1 and Grx2 with dithiol Grxs from other organisms. Strictly conserved residues and cysteines are depicted in boldface type. In addition, all cysteines are highlighted by a yellow background. Residues described as forming a groove for GSH binding on the surface of Grxs (10, 38, 39) or as undergoing, in addition to Cys30, large chemical shift changes in the NMR structure of Populus tremula GrxC4 upon titration with GSH (40) are shown with a blue background. Charged residues engaged in side chain interactions with a bound GSH molecule either in a mixed disulfide with mutants of E. coli Grx1 (38), human Grx1 (41), E. coli Grx3 (39), and yeast Grx2 (42) or in the non-covalent complex of human Grx2 with GSH (10) are highlighted by a red background. Those residues interacting with the glycine carboxylate of GSH are also underlined.