Introduction
This protocol describes methods for culturing Ae. aegypti. A procedure for egg collection is included and can be used in conjunction with the accompanying fixation, immunohistochemistry, and in situ protocols.
Materials
Equipment
Anesthetized mouse, rat, or chicken
Bowls (3 L)
Collected eggs
Cotton balls
Lids
Mosquito rearing cage
Paper towels
Petri dish (100 × 15 mm)
Plastic container with lid (approximately 1L size)
Plastic cups (500 ml)
Sealable sandwich bag
Strainer
Transfer pipettes
Reagents
Liver powder solution <R>
5% sucrose solution <R>
Method
I. Insectaries
The use of environmentally controlled chambers (insectaries) dedicated to mosquito rearing is encouraged. Adults can be maintained in mesh cages (Fig. 2) located in an insectary (see Christophers, 1960 for a discussion of cage construction). For general rearing, mosquitoes are maintained at 26°C, 84% relative humidity, under a 16 hr light and 8 hr dark cycle with 1 hr crepuscular periods at the beginning and end of each light cycle.
II. Life Cycle
The time in which a mosquito matures to adulthood is dependent upon environmental factors such as temperature, humidity, and nutrition. In the conditions described in this protocol, development proceeds roughly as follows:
Egg Hatch/First instar – days 1-2
Second instar – day 3
Third instar – day 4
Fourth instar – days 7-8 (males) or 8-9 (females)
Pupae – days 7-9
Adult – day 9 (males) or 10 (females)
III. Hatching Eggs
After ∼3 days, eggs in diapause that have been collected on egg paper (see below) can be used in scheduled rearing.
Fill a 500 ml plastic cup with 375 ml distilled water. Add 5 ml bovine liver powder suspension. Cut a piece of egg paper containing ∼300 eggs and place it in the cup. Avoid overcrowding during development as this will result in smaller mosquitoes.
After 1-2 days, larvae can be moved with a transfer pipette to a large (∼3 L) bowl containing ∼1.5 L water. Transfer ∼150 larvae per bowl, and again avoid overcrowding. Add ∼15 ml bovine liver powder suspension to each bowl and cover with a lid. Check the liver powder levels every 1-2 days. If all larvae are feeding in one area of the bowl, it is a good indication that an additional 5 ml of liver powder solution should be added.
-
Once larvae become pupae (there will be a mixture of larvae and pupae in the bowl), transfer the pupae from the bowl into a 500 ml plastic cup containing 250 ml distilled water, which will be placed into a rearing cage. For the transfer, you should:
Gather the bowl that contains pupae/larvae, two additional clean/empty bowls, two cups, distilled water, a transfer pipette, and a strainer for catching pupae/larvae. Mosquito rearing cages should be prepared ahead of time.
Pour the contents of the bowl containing pupae/larvae through a strainer into an empty bowl. The animals will remain in the strainer; the water collected in the bowl should be saved, as you will eventually need to transfer the larvae back into this water.
Rinse the animals by slightly submerging the strainer in distilled water. Shake slightly to remove liver powder. Empty the animals into a clean bowl containing distilled water.
Once all animals have been collected, use a transfer pipette to relocate only the pupae into a 500 ml cup containing 250 ml distilled water. Pour the remaining larvae through the strainer, and transfer them back into the bowl containing your original water. Add bovine liver powder suspension if necessary.
Place the cup containing pupae inside of a rearing cage and allow the pupae to become adults. You may need to split the pupae into a few cups or cages depending on the number collected (we aim for ∼125 per 20×20×30 cm cage).
In late afternoon the following day, collect any additional pupae that have developed and add them to the cage. Soak 3-4 cotton balls in 5% sucrose solution, squeeze them together slightly to make one ball, and place it in the mosquito rearing cage.
IV. Blood Feeding
Protocols for use of animals for blood feeding must be approved by the appropriate Institutional Animal Care and Use Committee. Our protocols typically use anesthetized mice or rats depending on numbers of mosquitoes to be blood fed. For blood feeding, animals should be placed on of the top of the netted cage for ∼15 min. Adult female mosquitoes should be aged ∼3 days and should be deprived of sucrose solution ∼12-24 hr prior to blood feeding.
V. Egg Collection
a. Collection of eggs for basic colony maintenance
Egg collections can be made ∼3 days following blood feeding, at which time each adult female will lay ∼100-150 eggs. The following procedure will allow for collection of eggs that will be used for maintenance of the culture. The procedure differs slightly for timed collections (see V.b) or when collecting eggs for microinjection (see microinjection protocol).
Cut a piece of paper towel (∼9 cm × 20 cm). Label it with the strain type, date, and time.
Get a 500 ml plastic cup and add ∼250 ml distilled water. Place the paper towel in direct contact with the inner wall of the cup (make sure writing faces cup wall) and along the water/air interface. Place the cup in a cage.
Allow the mosquitoes to lay eggs for ∼3 days (a more narrow window can be used if staged collections are desired, see below). After this, collect the paper towel, hereafter referred to as egg paper. Mosquitoes will typically lay the majority of their eggs within one day of egg paper placement. Keep the egg paper in the cup for an additional 24 hrs, and then remove it for drying.
Cover the egg paper and allow it to dry for ∼3 days in the insectary. Once dry, wrap it with a piece of folded paper towel and place it in a plastic container, where it can then be stored for up to 3 months. During this extended dessication period, development will arrest at the first larval stage. When eggs from such collections are placed in water, animals will hatch as first instar larvae over the next few hours.
b. Collection of embryos at a particular developmental stage
If you wish to obtain embryos synchronized at a particular stage of embryonic development, use a narrower egg laying time (i.e. 30 min.). Following egg collection, place the egg paper onto a layer of wet paper towels, and put the egg paper/paper towels into a sealable sandwich bag or plastic container. Keep the bag in the insectary or in an incubator set at 26° C. Be sure that the egg paper remains damp (but not soaking wet) as you age the embryos to the desired stage.
Troubleshooting
Problem: Slow development (II. Life cycle); Solution: Overcrowding can slow development, as larvae will not be able to acquire adequate nutrition. Adhere to the cage densities and feeding instructions described throughout this protocol.
Problem: Poor hatching (step III.1); Solution: Do not store eggs more than three months. The time required for eggs to hatch will increase and egg viability will decrease as you approach the three month mark.
Problem: Variable egg-laying (step V); Solutions: For basic colony maintenance, prepare fresh cages every two weeks. Be sure to blood feed the mosquitoes weekly when you need to perform egg collections. Be aware that mosquitoes will lay the majority of their eggs on the first egg paper placed following a blood feed. This makes it difficult to collect eggs in a single cage of mosquitoes more than one time following a single blood meal. To combat this problem, it can be helpful to rear multiple cages of mosquitoes. It is also possible to transfer a small number of blood fed females from a large cage to a smaller egg collection chamber; females remaining in the larger cage can then be used for later egg collections (see Lobo et al., 2006).
Discussion
The culturing methodology described here allows for fairly routine maintenance of Ae. aegypti. The procedure for egg-laying can be used to collect large numbers of eggs for in situ hybridization analysis of gene expression or immunohistemical analysis of protein expression, both of which are revealing new insight into the development of this vector mosquito (Simanton et al., 2009).
Fig. 1. Mosquito rearing cage.
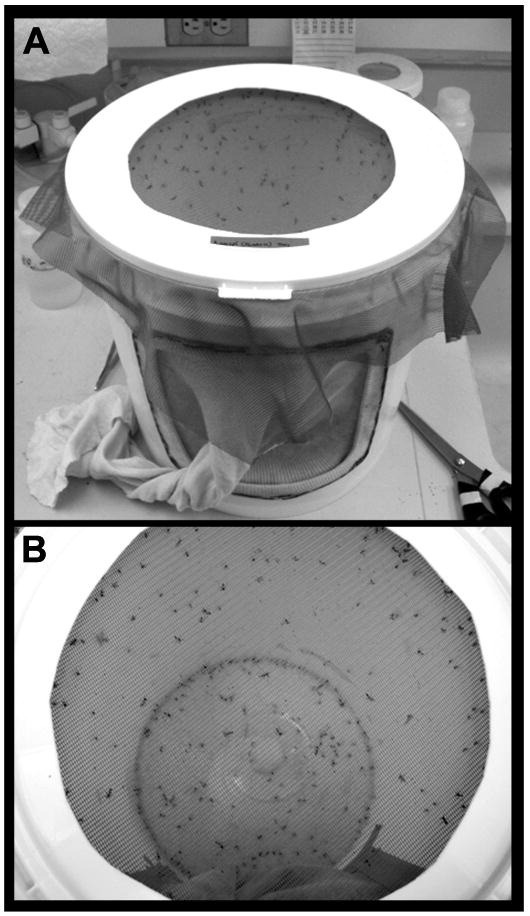
The cotton sleeve on the cage (seen in A) permits human access and manipulations within the cage. The netted top of the cage (top down view shown in B) allows for blood feeding prior to egg collections.
Acknowledgments
Development of the protocols described here was funded by the following awards NIH/NIAID Award R01 AI 081795, NIH/NINDS Award R15 NS 048904, and an IUSM Research Support Funds Grant to MDS and NIH/NIAID Award RO1 AI 059342 to DWS.
Recipes
Bovine Liver Powder Suspension
Take a squirt bottle and trim the tip to create a larger opening. Weigh 5 g of liver powder (MP Biomedicals Cat# 900396) and add dH20 to a final volume of 500 ml. Mix and store at 4° C. Shake well before each use.
Sucrose Solution (5%)
Combine 9.5 g sucrose with 189 ml distilled water. Mix the reagents and store at 4° C.
Footnotes
Conflicts of interest: none declared
References
- Christophers SR. Aedes aegypti, The yellow fever mosquito: its life history, bionomics, and structure. Cambridge University Press; Cambridge, UK: 1960. [Google Scholar]
- Lobo NF, Clayton JR, Fraser MJ, Kafatos FC, Collins FH. High efficiency germ-line transformation of mosquitoes. Nat Protoc. 2006;1:1312–1317. doi: 10.1038/nprot.2006.221. [DOI] [PubMed] [Google Scholar]
- Simanton W, Clark S, Clemons A, Jacowski C, Farrell-VanZomeren A, Beach P, Browne WE, Duman-Scheel M. Conservation of arthropod midline netrin accumulation revealed with a cross-reactive antibody provides evidence for midline cell homology. Evol Dev. 2009;11:260–268. doi: 10.1111/j.1525-142X.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


