Abstract
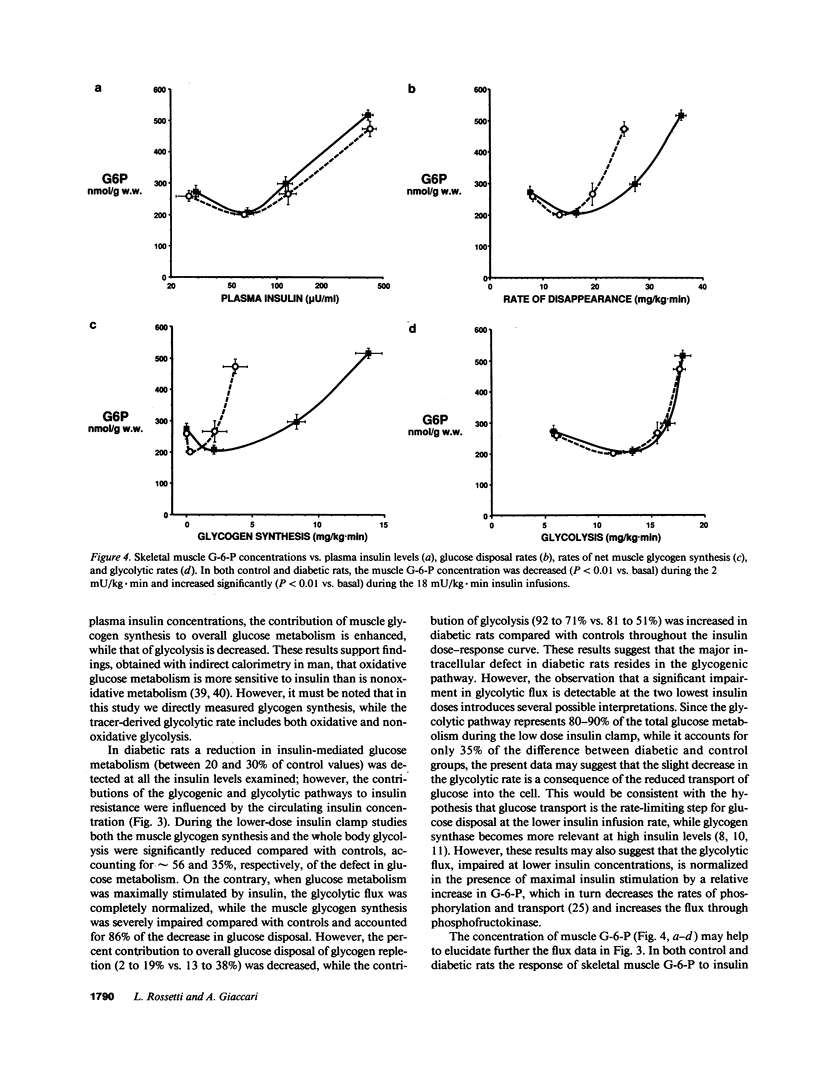
To examine the relationship between plasma insulin concentration and intracellular glucose metabolism in control and diabetic rats, we measured endogenous glucose production, glucose uptake, whole body glycolysis, muscle and liver glycogen synthesis, and rectus muscle glucose-6-phosphate (G-6-P) concentration basally and during the infusion of 2, 3, 4, 12, and 18 mU/kg.min of insulin. The contribution of glycolysis decreased and that of muscle glycogen synthesis increased as the insulin levels rose. Insulin-mediated glucose disposal was decreased by 20-30% throughout the insulin dose-response curve in diabetics compared with controls. While at low insulin infusions (2 and 3 mU/kg.min) reductions in both the glycolytic and glycogenic fluxes contributed to the defective tissue glucose uptake in diabetic rats, at the three higher insulin doses the impairment in muscle glycogen repletion accounted for all of the difference between diabetic and control rats. The muscle G-6-P concentration was decreased (208 +/- 11 vs. 267 +/- 18 nmol/g wet wt; P less than 0.01) compared with saline at the lower insulin infusion, but was gradually increased twofold (530 +/- 16; P less than 0.01 vs. basal) as the insulin concentration rose. The G-6-P concentration in diabetic rats was similar to control despite the reduction in glucose uptake. These data suggest that (a) glucose transport is the major determinant of glucose disposal at low insulin concentration, while the rate-limiting step shifts to an intracellular site at high physiological insulin concentration; and (b) prolonged moderate hyperglycemia and hypoinsulinemia determine two distinct cellular defects in skeletal muscle at the levels of glucose transport/phosphorylation and glycogen synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Weir G. C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983 Jun;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Hendler R., Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes. 1982 Sep;31(9):795–801. doi: 10.2337/diab.31.9.795. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Tapscott E. B., Pories W. J., Dabbs D. J., Flickinger E. G., Meelheim D., Fushiki T., Atkinson S. M., Elton C. W., Caro J. F. An in vitro human muscle preparation suitable for metabolic studies. Decreased insulin stimulation of glucose transport in muscle from morbidly obese and diabetic subjects. J Clin Invest. 1988 Aug;82(2):486–494. doi: 10.1172/JCI113622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Smith J. D., Cobelli C., Toffolo G., Pilo A., DeFronzo R. A. Effect of insulin on the distribution and disposition of glucose in man. J Clin Invest. 1985 Jul;76(1):357–364. doi: 10.1172/JCI111969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. E., Huecksteadt T. P. Glucose 6-phosphate effects on deoxyglucose, glucose and methylglucose transport in rat adipocytes. Evidence for intracellular regulation of sugar transport by glucose metabolites. Biochim Biophys Acta. 1984 Nov 13;805(3):313–316. doi: 10.1016/0167-4889(84)90088-0. [DOI] [PubMed] [Google Scholar]
- Garvey W. T., Huecksteadt T. P., Matthaei S., Olefsky J. M. Role of glucose transporters in the cellular insulin resistance of type II non-insulin-dependent diabetes mellitus. J Clin Invest. 1988 May;81(5):1528–1536. doi: 10.1172/JCI113485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboe D. P., Nuttall F. Q. The role of ATP and glucose 6-phosphate in the regulation of glycogen synthetase D phosphatase. Biochem Biophys Res Commun. 1972 Aug 21;48(4):898–906. doi: 10.1016/0006-291x(72)90693-6. [DOI] [PubMed] [Google Scholar]
- Gottesman I., Mandarino L., Verdonk C., Rizza R., Gerich J. Insulin increases the maximum velocity for glucose uptake without altering the Michaelis constant in man. Evidence that insulin increases glucose uptake merely by providing additional transport sites. J Clin Invest. 1982 Dec;70(6):1310–1314. doi: 10.1172/JCI110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen I. L., Cryer P. E., Rizza R. A. Comparison of insulin-mediated and glucose-mediated glucose disposal in patients with insulin-dependent diabetes mellitus and in nondiabetic subjects. Diabetes. 1985 Aug;34(8):751–755. doi: 10.2337/diab.34.8.751. [DOI] [PubMed] [Google Scholar]
- Hollenbeck C. B., Chen Y. D., Reaven G. M. A comparison of the relative effects of obesity and non-insulin-dependent diabetes mellitus on in vivo insulin-stimulated glucose utilization. Diabetes. 1984 Jul;33(7):622–626. doi: 10.2337/diab.33.7.622. [DOI] [PubMed] [Google Scholar]
- Hom F. G., Goodner C. J., Berrie M. A. A [3H]2-deoxyglucose method for comparing rates of glucose metabolism and insulin responses among rat tissues in vivo. Validation of the model and the absence of an insulin effect on brain. Diabetes. 1984 Feb;33(2):141–152. doi: 10.2337/diab.33.2.141. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Landau B. R. Estimation of the pentose cycle contribution to glucose metabolism in tissue in vivo. Biochemistry. 1967 Oct;6(10):2961–2964. doi: 10.1021/bi00862a001. [DOI] [PubMed] [Google Scholar]
- Karlander S., Roovete A., Vranić M., Efendić S. Glucose and fructose 6-phosphate cycle in humans. Am J Physiol. 1986 Nov;251(5 Pt 1):E530–E536. doi: 10.1152/ajpendo.1986.251.5.E530. [DOI] [PubMed] [Google Scholar]
- Katz A., Nyomba B. L., Bogardus C. No accumulation of glucose in human skeletal muscle during euglycemic hyperinsulinemia. Am J Physiol. 1988 Dec;255(6 Pt 1):E942–E945. doi: 10.1152/ajpendo.1988.255.6.E942. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraegen E. W., James D. E., Bennett S. P., Chisholm D. J. In vivo insulin sensitivity in the rat determined by euglycemic clamp. Am J Physiol. 1983 Jul;245(1):E1–E7. doi: 10.1152/ajpendo.1983.245.1.E1. [DOI] [PubMed] [Google Scholar]
- Kubo K., Foley J. E. Rate-limiting steps for insulin-mediated glucose uptake into perfused rat hindlimb. Am J Physiol. 1986 Jan;250(1 Pt 1):E100–E102. doi: 10.1152/ajpendo.1986.250.1.E100. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Zawadzki J. K., Young A. A., Abbott W. G., Bogardus C. Glucose storage is a major determinant of in vivo "insulin resistance" in subjects with normal glucose tolerance. J Clin Endocrinol Metab. 1986 May;62(5):922–927. doi: 10.1210/jcem-62-5-922. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Madar Z., Kolterman O. G., Bell J. M., Olefsky J. M. Adipocyte glycogen synthase and pyruvate dehydrogenase in obese and type II diabetic subjects. Am J Physiol. 1986 Oct;251(4 Pt 1):E489–E496. doi: 10.1152/ajpendo.1986.251.4.E489. [DOI] [PubMed] [Google Scholar]
- Mandarino L. J., Wright K. S., Verity L. S., Nichols J., Bell J. M., Kolterman O. G., Beck-Nielsen H. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest. 1987 Sep;80(3):655–663. doi: 10.1172/JCI113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Okubo M., Bogardus C., Lillioja S., Mott D. M. Glucose-6-phosphate stimulation of human muscle glycogen synthase phosphatase. Metabolism. 1988 Dec;37(12):1171–1176. doi: 10.1016/0026-0495(88)90196-5. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Lauglin M. R. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J Clin Invest. 1989 Sep;84(3):892–899. doi: 10.1172/JCI114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L. Normalization of insulin sensitivity with lithium in diabetic rats. Diabetes. 1989 May;38(5):648–652. doi: 10.2337/diab.38.5.648. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Rothman D. L., DeFronzo R. A., Shulman G. I. Effect of dietary protein on in vivo insulin action and liver glycogen repletion. Am J Physiol. 1989 Aug;257(2 Pt 1):E212–E219. doi: 10.1152/ajpendo.1989.257.2.E212. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I., Rossetti L., Rothman D. L., Blair J. B., Smith D. Quantitative analysis of glycogen repletion by nuclear magnetic resonance spectroscopy in the conscious rat. J Clin Invest. 1987 Aug;80(2):387–393. doi: 10.1172/JCI113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. T., Koudelka A. P. Pathway of glycogen synthesis from glucose in hepatocytes maintained in primary culture. J Biol Chem. 1985 Feb 10;260(3):1521–1526. [PubMed] [Google Scholar]
- Young A. A., Bogardus C., Wolfe-Lopez D., Mott D. M. Muscle glycogen synthesis and disposition of infused glucose in humans with reduced rates of insulin-mediated carbohydrate storage. Diabetes. 1988 Mar;37(3):303–308. doi: 10.2337/diab.37.3.303. [DOI] [PubMed] [Google Scholar]
- Ziel F. H., Venkatesan N., Davidson M. B. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988 Jul;37(7):885–890. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]



