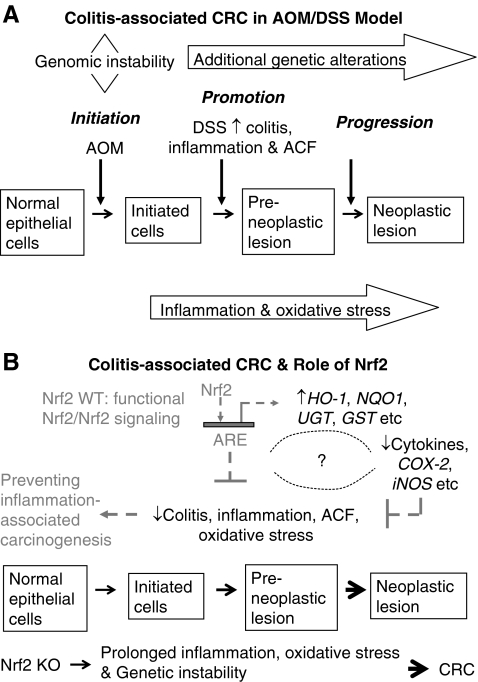
FIG. 6.
A simplified illustration shows the carcinogenesis in human CRC and the role of Nrf2 in antiinflammation and oxidative stress. (A) Pathways that connect inflammation and CRC in AOM/DSS animal model, a chemical-carcinogen-induced CRC model. (B) Role of Nrf2 in protecting inflammation-associated cancer, showing a potential strategy for prevention of inflammation-associated cancers. When the Nrf2 signaling pathway is activated, activation of expression of phase II antioxidant and detoxifying enzymes such as HO-1, NQO1, UGT, and GST alike in Nrf2 WT animals can reduce the oxidative stress, and it is postulated that Nrf2 could also regulate proinflammatory genes and cytokines such as iNOS, COX-2, and iL-6 alike, and together, they protect the animal from developing inflammation-associated cancer, such as CRC (broken arrows). In contrast, Nrf2 KO animals do not possess functional Nrf2 and Nrf2 signaling pathway; after prolonged inflammatory and oxidative stress caused by AOM/DSS, they have increased incidence, multiplicity, and size of all colorectal tumors, including adenoma and adenocarcinoma as compared with WT animals (solid arrows). ACF, aberrant crypt foci; AOM, azoxymethane; CRC, colorectal cancer; DSS, dextran sulfate sodium; GST, glutathione S-transferase; HO-1, heme oxygenase-1; Nrf2, NF-E2-related factor 2; Nrf2 KO, Nrf2 knockout; Nrf2 WT mice, wild-type mice with intact Nrf2 function; NQO1, NAD(P)H:quinone oxidoreductase 1; UGT, UDP-glucuronosyltransferase.