Table 1.
Microwave-assisted one-pot TMS-deprotection of compounds 1a-g and cycloaddition with azides 2a-c to give triazoles 3a-i
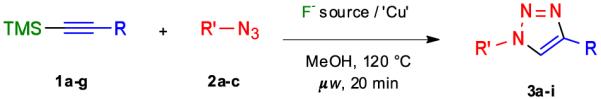 | ||||
|---|---|---|---|---|
| entry | R | R’ | method Aa yield (%) |
method Bb yield (%) |
| 1 | C6H5 | Bn | 98 | 98c |
| 2 | 4-MeO-C6H4 | Bn | 97 | 96 |
| 3 | 4-CF3-C6H4 | Bn | 98 | 97 |
| 4 | 4-Cl-C6H4 | Bn | 89 | 95 |
| 5 | 4-Br-C6H4 | Bn | 81 | 91 |
| 6 | 2-Br-C6H4 | Bn | 89 | 90 |
| 7 | CH2OH | Bn | 82 | 98 |
| 8 | C6H5 | 4-MeO-Bn | 87 | 98 |
| 9 | C6H5 | 4-NO2-Bn | 98 | 99 |
A mixture of TBAF (1.0 M, 2.0 equiv) and CuI (10 mol %) and DIPEA (20 mol %) was used
CuF2 (2.0 equiv) was used
3a was isolated in yields of 22% and 57% when the reaction was performed at 60 °C or 90 °C, respectively.
