Table 2.
One-pot, three-step synthesis of 1,4-disubstituted triazoles 3
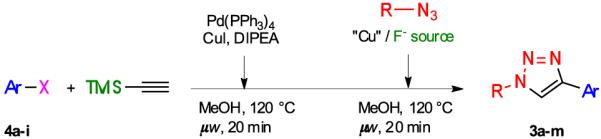 | ||||
|---|---|---|---|---|
| entry | Ar-X | R | method Aa yield (%) |
method Bb yield (%) |
| 1 | Ph-I | Bn | 96 | 91 |
| 2 | 4-MeO-C6H4-I | Bn | 41 | 54 |
| 3 | 4-CF3-C6H4-I | Bn | 97 | 98 |
| 4 | 4-F-C6H4-I | Bn | 98 | 98 |
| 5 | 4-Me-C6H4-I | Bn | 93 | 95 |
| 6 | 2-Me-C6H4-I | Bn | 97 | 98 |
| 7 | Ph-Br | Bn | 42 | 65 |
| 8 | 4-MeO-C6H4-Br | Bn | 13 | 15(57c) |
| 9 | 4-NO2-C6H4-Br | Bn | 12 | 11(53c) |
| 10 | Ph-I | 4-MeO-Bn\ | 84 | 91 |
| 11 | Ph-I | 4-NO2-Bn | 97 | 98 |
A mixture of TBAF (1.0 M, 2.0 equiv) and CuI (10 mol %) was used
CuF2 (2.0 equiv) was used
The Sonogashira cross coupling was performed using Pd(PPh3)2Cl2 (5 mol %), CuI (10 mol %) in refluxing Et3N for 10 h.
