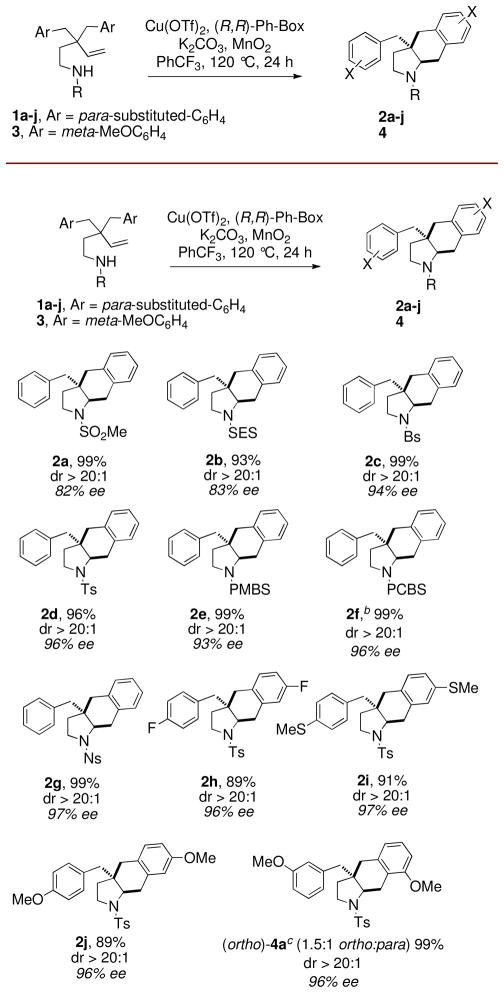
Figure 1.
aEnantioselective Carboamination Scope. a20 mol % Cu(OTf)2 and 25 mol % (R,R)-Ph-Box were combined in PhCF3 (0.1 M w/r to 1) and heated at 60 °C for 2 h in a pressure tube, then 1, K2CO3 (100 mol %), MnO2 (300 mol %) were added and the reaction was heated at 120 °C for 24 h. Yield refers to product isolated from flash chromatography on SiO2. Enantioselectivity (%ee) was determined by chiral HPLC. bReaction run at 110 °C. cYield is for combined regioisomeric mixture, dr and %ee were the same for both isomers. SES = trimethylsilylethlysulfonyl, Bs = benzenesulfonyl, PMBS = 4-methoxybenzenesulfonyl, PCBS = 4-chlorobenzenesulfonyl, Ns = 4-nitrobenzenesulfonyl.