Table 2.
Cross-Coupling of 4a with Diverse Aryl Chlorides
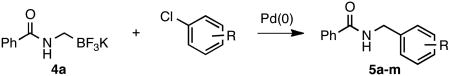 | ||||
|---|---|---|---|---|
| entry | chloride | product | % isolated yield | |
| 1 | 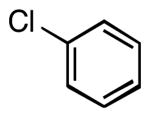 |
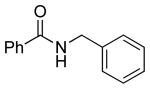 |
5a | 87 |
| 2 | 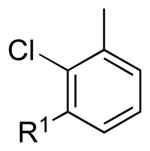 |
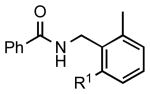 |
5b: R1 = H | 83 (91)a |
| 5c: R1 = Me | 88b | |||
| 3 | 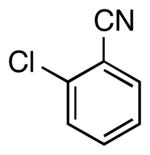 |
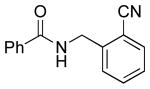 |
5d | 65a |
| 4 | 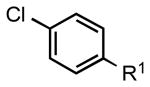 |
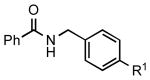 |
5e: R1 = CN | 87 |
| 5f: R1 = CHO | 88 | |||
| 5g: R1 = Ac | 88 | |||
| 5 | 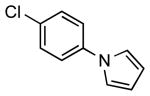 |
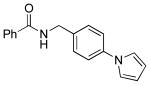 |
5h | 98 |
| 6 | 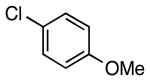 |
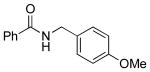 |
5i | 95 (91)c |
| 7 | 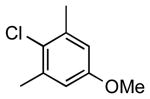 |
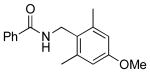 |
5j | 74 |
| 8 | 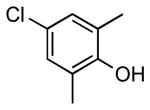 |
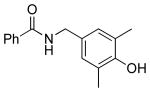 |
5k | 87 |
| 9 | 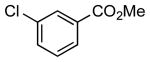 |
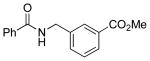 |
5l | 89 |
| 10 | 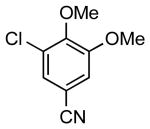 |
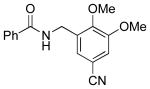 |
5m | 76 |
All reactions were carried out using 0.3 mmol of 4a and aryl chloride, 2.5 mol % Pd(OAc)2, 5 mol % XPhos, 0.9 mmol of Cs2CO3, 10:1 CPME/H2O (0.09 M), 85 °C, 6 h.
Used 5 mol % Pd(OAc)2, 10 mol % XPhos.
Heated reaction for 14 h.
Reaction performed on 4.1 mmol scale using 1 mol % Pd(OAc)2 and 2 mol % XPhos, 24 h at 85 °C.
