Abstract
In previous studies, IL-4 has been reported to interfere with IL-2-driven generation of lymphokine-activated killer (LAK) activity. In this investigation, we have demonstrated that IL-4 inhibited the IL-2-induced differentiation of large granular lymphocytes (LGL) into LAK effectors by a mechanism involving, at least in part, an increase in LGL intracellular cAMP levels. In contrast, with its capacity to induce cAMP accumulation in resting LGL, IL-4 had a very negligible effect on LAK activity induction, and cAMP levels increase in LGL that had been preincubated with IL-2. Furthermore, the inhibitory effect of IL-4 on LAK activity generation also correlated with a marked decrease in N-CBZ-L-lysine thiobenzylester esterase activity, with an inhibition of tumor necrosis factor (TNF) mRNA expression and TNF production by IL-2-stimulated LGL. These results strongly suggest that complex signaling processes could be ascribed to the dual activities of cytokines and their interplay in LAK promotion.
Full text
PDF
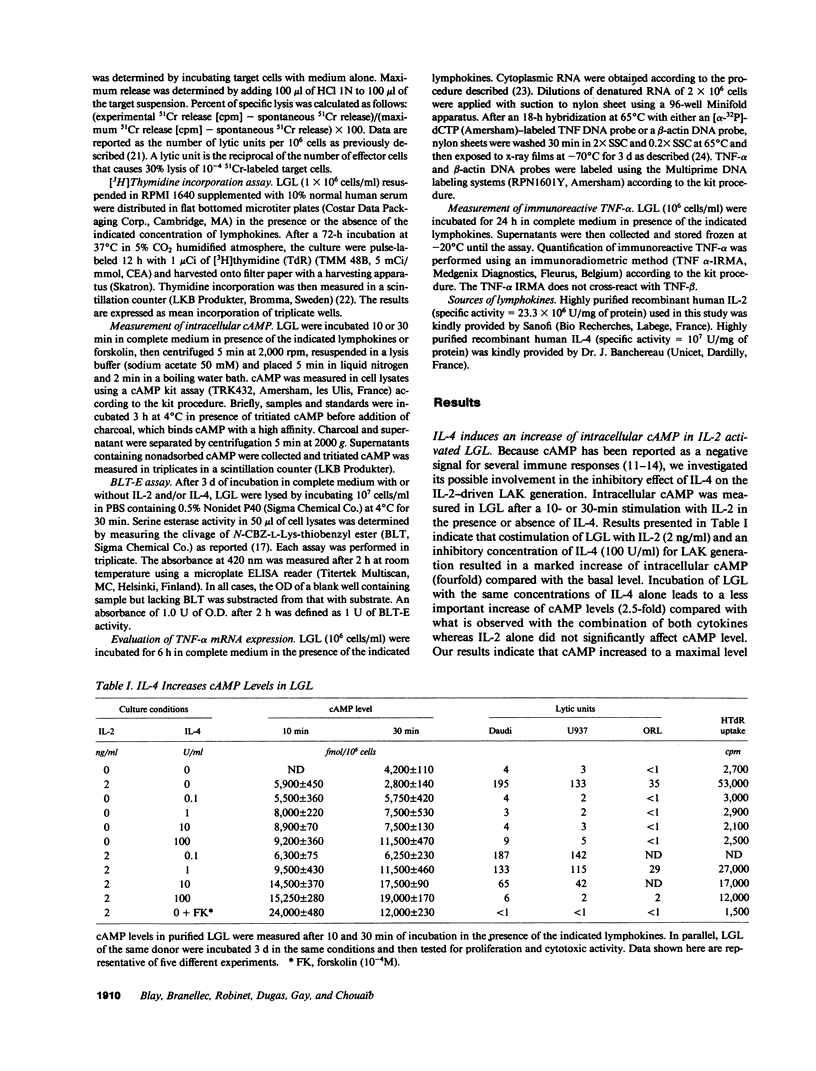

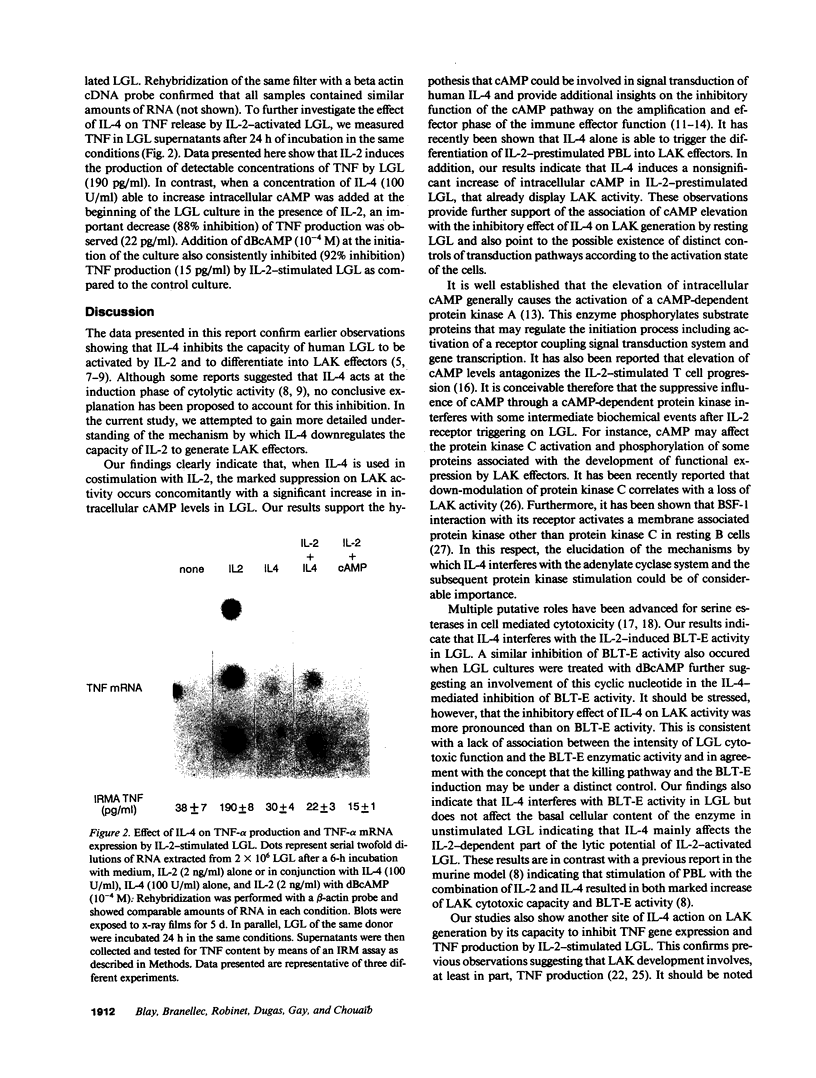

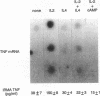
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckner S. K., Farrar W. L. Potentiation of lymphokine-activated killer cell differentiation and lymphocyte proliferation by stimulation of protein kinase C or inhibition of adenylate cyclase. J Immunol. 1988 Jan 1;140(1):208–214. [PubMed] [Google Scholar]
- Blay J. Y., Bertoglio J., Fradelizi D., Chouaib S. Functional interactions of IL2 and TNF in the differentiation of LGL into LAK effectors. Int J Cancer. 1989 Oct 15;44(4):598–604. doi: 10.1002/ijc.2910440407. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Bertoglio J., Blay J. Y., Marchiol-Fournigault C., Fradelizi D. Generation of lymphokine-activated killer cells: synergy between tumor necrosis factor and interleukin 2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6875–6879. doi: 10.1073/pnas.85.18.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaib S., Robb R. J., Welte K., Dupont B. Analysis of prostaglandin E2 effect on T lymphocyte activation. Abrogation of prostaglandin E2 inhibitory effect by the tumor promotor 12.0 tetradecanoyl phorbol-13 acetate. J Clin Invest. 1987 Aug;80(2):333–340. doi: 10.1172/JCI113077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degliantoni G., Murphy M., Kobayashi M., Francis M. K., Perussia B., Trinchieri G. Natural killer (NK) cell-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor. Relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med. 1985 Nov 1;162(5):1512–1530. doi: 10.1084/jem.162.5.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Botran R., Krammer P. H., Diamantstein T., Uhr J. W., Vitetta E. S. B cell-stimulatory factor 1 (BSF-1) promotes growth of helper T cell lines. J Exp Med. 1986 Aug 1;164(2):580–593. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. W., Davis B. H., Smith K. A. cAMP antagonizes interleukin 2-promoted T-cell cycle progression at a discrete point in early G1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6072–6076. doi: 10.1073/pnas.85.16.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justement L., Chen Z., Harris L., Ransom J., Sandoval V., Smith C., Rennick D., Roehm N., Cambier J. BSF1 induces membrane protein phosphorylation but not phosphoinositide metabolism, Ca2+ mobilization, protein kinase C translocation, or membrane depolarization in resting murine B lymphocytes. J Immunol. 1986 Dec 1;137(11):3664–3670. [PubMed] [Google Scholar]
- Kammer G. M. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988 Jul-Aug;9(7-8):222–229. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Custer M. C., Rosenberg S. A., Lotze M. T. IL-4 regulates IL-2 induction of lymphokine-activated killer activity from human lymphocytes. J Immunol. 1989 May 15;142(10):3452–3461. [PubMed] [Google Scholar]
- Manyak C. L., Norton G. P., Lobe C. G., Bleackley R. C., Gershenfeld H. K., Weissman I. L., Kumar V., Sigal N. H., Koo G. C. IL-2 induces expression of serine protease enzymes and genes in natural killer and nonspecific T killer cells. J Immunol. 1989 May 15;142(10):3707–3713. [PubMed] [Google Scholar]
- Mosmann T. R., Bond M. W., Coffman R. L., Ohara J., Paul W. E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulé J. J., Krosnick J. A., Rosenberg S. A. IL-4 regulation of murine lymphokine-activated killer activity in vitro. Effects on the IL-2-induced expansion, cytotoxicity, and phenotype of lymphokine-activated killer effectors. J Immunol. 1989 Jan 15;142(2):726–733. [PubMed] [Google Scholar]
- Mulé J. J., Smith C. A., Rosenberg S. A. Interleukin 4 (B cell stimulatory factor 1) can mediate the induction of lymphokine-activated killer cell activity directed against fresh tumor cells. J Exp Med. 1987 Sep 1;166(3):792–797. doi: 10.1084/jem.166.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T., Burakoff S. J., Herrmann S. H. Inhibition of lymphokine-activated killer cell-mediated cytotoxicity by phorbol ester. J Immunol. 1989 Mar 15;142(6):2155–2161. [PubMed] [Google Scholar]
- Ostergaard H. L., Kane K. P., Mescher M. F., Clark W. R. Cytotoxic T lymphocyte mediated lysis without release of serine esterase. Nature. 1987 Nov 5;330(6143):71–72. doi: 10.1038/330071a0. [DOI] [PubMed] [Google Scholar]
- Pasternack M. S., Eisen H. N. A novel serine esterase expressed by cytotoxic T lymphocytes. 1985 Apr 25-May 1Nature. 314(6013):743–745. doi: 10.1038/314743a0. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Yssel H., Paliard X., Kastelein R., Figdor C., de Vries J. E. IL-4 inhibits IL-2-mediated induction of human lymphokine-activated killer cells, but not the generation of antigen-specific cytotoxic T lymphocytes in mixed leukocyte cultures. J Immunol. 1988 Jul 1;141(1):29–36. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenn G., Takayama H., Sitkovsky M. V. Exocytosis of cytolytic granules may not be required for target cell lysis by cytotoxic T-lymphocytes. Nature. 1987 Nov 5;330(6143):72–74. doi: 10.1038/330072a0. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Widmer M. B., Acres R. B., Sassenfeld H. M., Grabstein K. H. Regulation of cytolytic cell populations from human peripheral blood by B cell stimulatory factor 1 (interleukin 4). J Exp Med. 1987 Nov 1;166(5):1447–1455. doi: 10.1084/jem.166.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Otsuka T., Mosmann T., Banchereau J., DeFrance T., Blanchard D., De Vries J. E., Lee F., Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]