Abstract
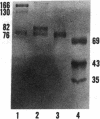
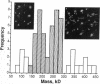
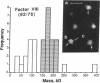
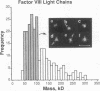
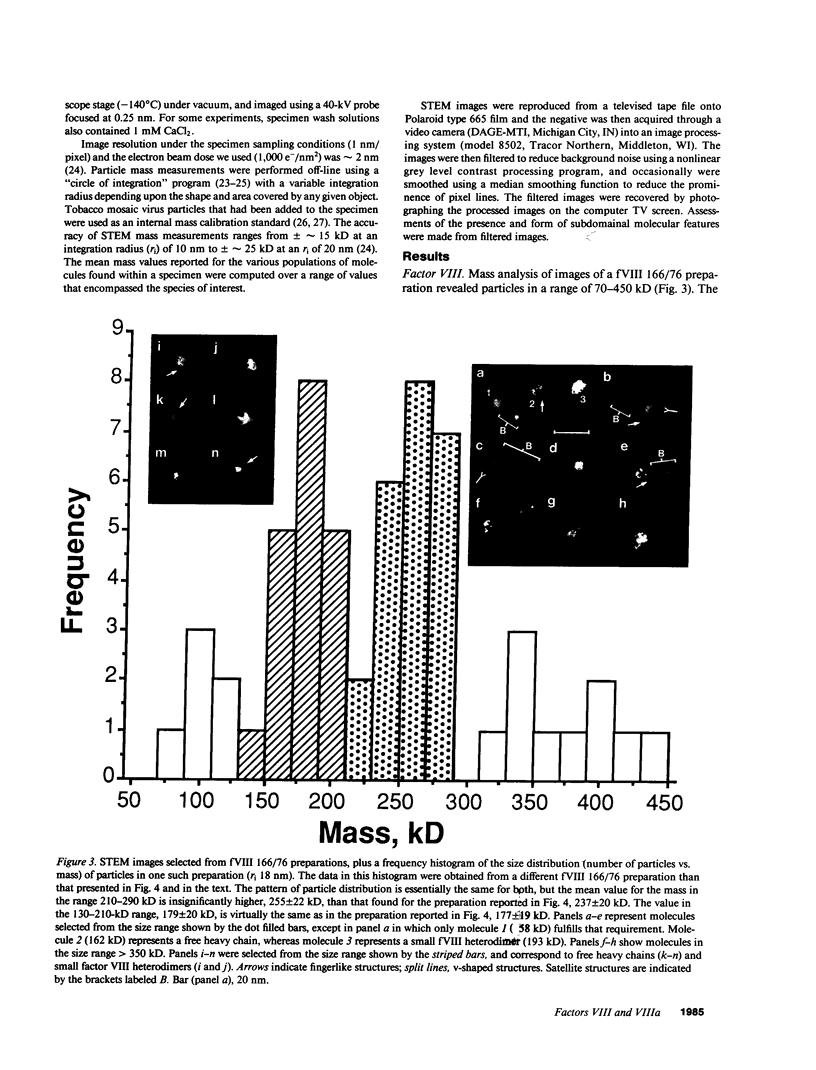
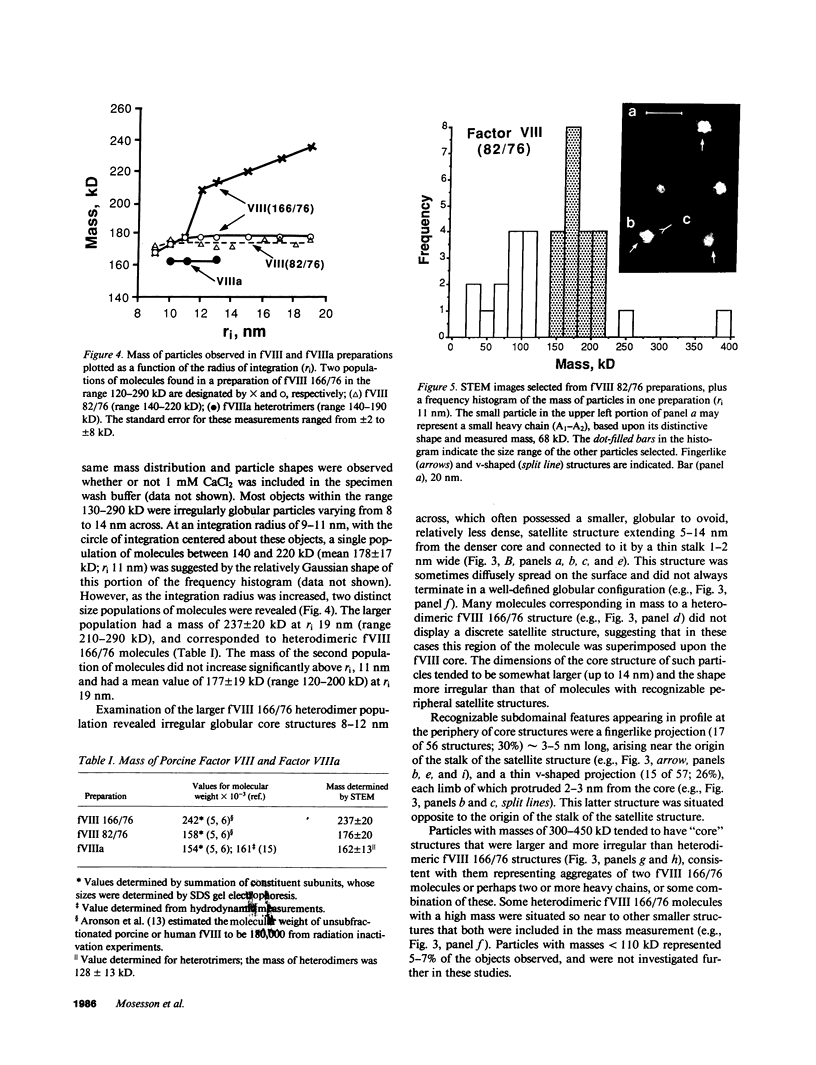
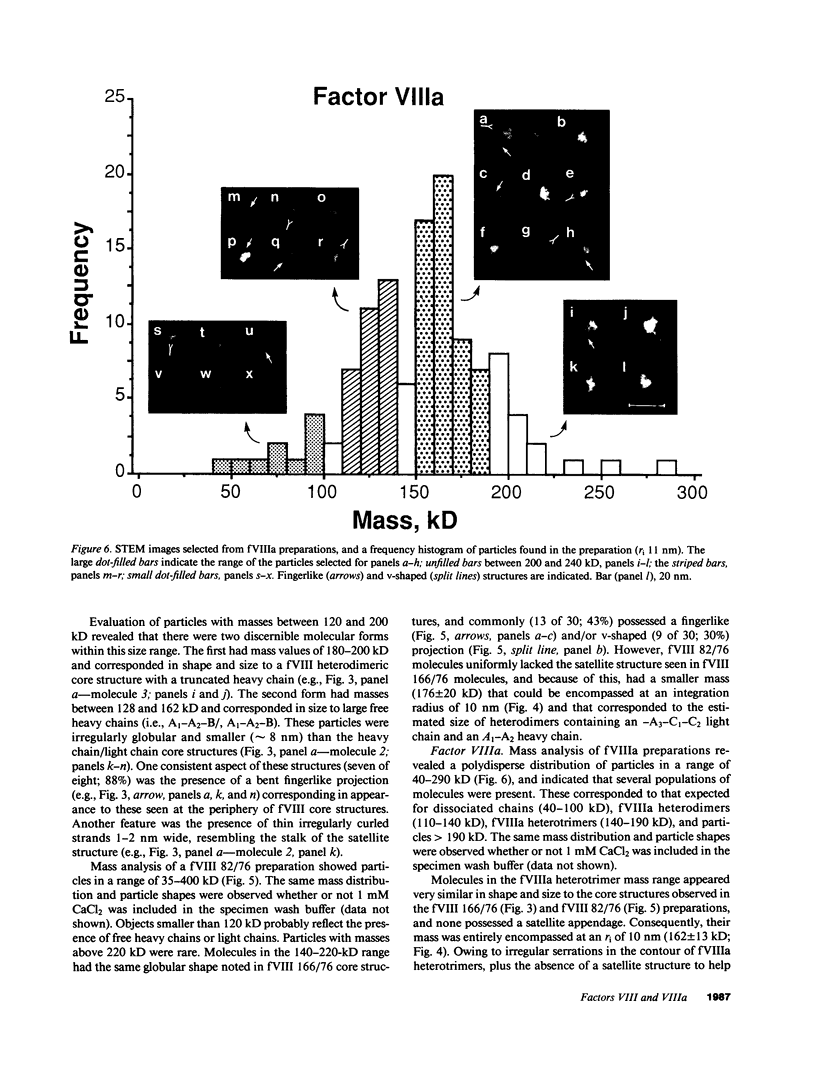
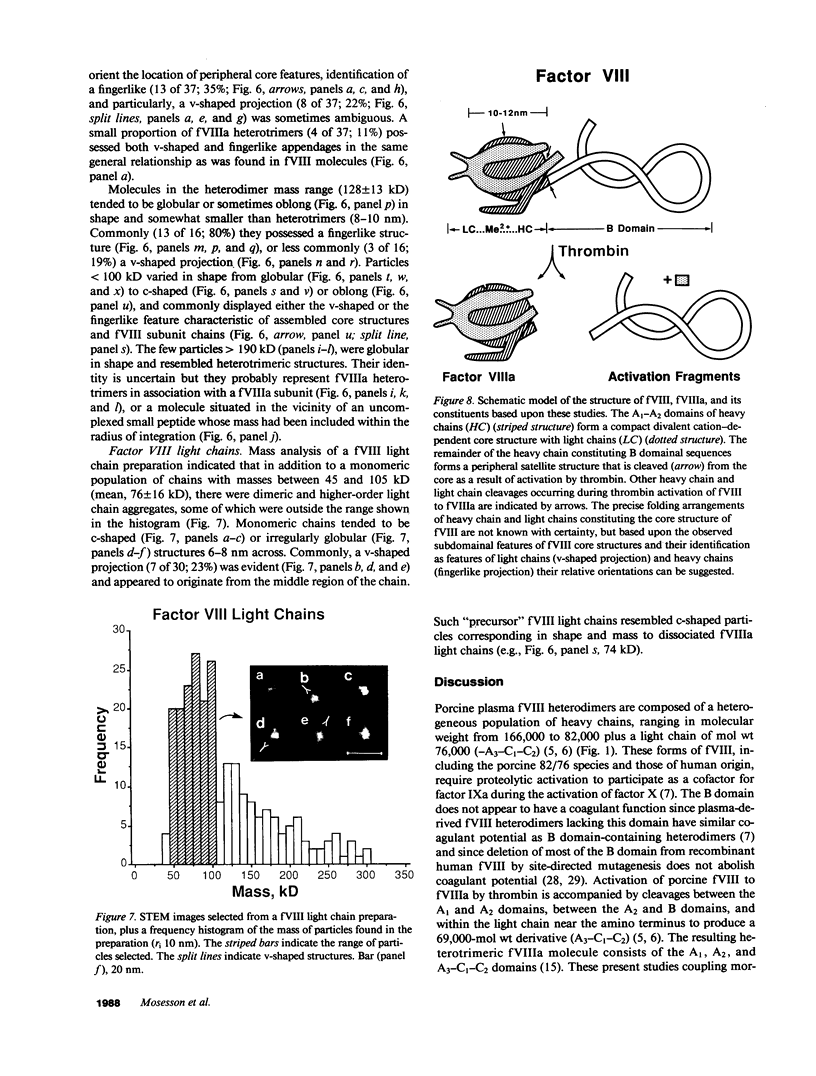
Porcine plasma factor VIII (fVIII) molecules are heterodimers composed of a 76,000-mol wt light chain (-A3-C1-C2) and a heavy chain ranging in molecular weight from 82,000 (A1-A2) to 166,000 (A1-A2-B). Proteolytic activation of fVIII by thrombin results in fVIIIa heterotrimers lacking B domains (A1, A2, A3-C1-C2). In this study, immunoaffinity purified fVIII was further fractionated by mono S or mono Q chromatography to prepare heterodimers containing a light chain and an A1-A2-B heavy chain (fVIII 166/76) or an A1-A2 heavy chain (fVIII 82/76). Mass analysis of scanning transmission electron microscopic (STEM) images of fVIII 166/76 indicated that heterodimers (mass 237 +/- 20 kD) had irregularly globular core structures 10-12 nm across, and frequently displayed a diffuse, occasionally globular to ovoid satellite structure extending 5-14 nm from the core, and attached to it by a thin stalk. Factor VIII 82/76 molecules (mass 176 +/- 20 kD) had the same core structures as fVIII 166/76 molecules, but lacked the satellite structure. These findings indicate that A1-A2 domains of heavy chains and the light chains of the fVIII procofactor molecule are closely associated and constitute the globular core structure, whereas the B domainal portion of heavy chains comprises the peripheral satellite appendage. Factor VIII core structures commonly displayed a finger-like projection near the origin of the B domainal stalk that was also a consistent feature of the free heavy chains (mass 128-162 kD) found in fVIII 166/76 preparations. Factor VIII light chain monomers (mass, 76 +/- 16 kD) were globular to c-shaped particles 6-8 nm across. These chains commonly possessed a v-shaped projection originating from its middle region, that could also be observed at the periphery of fVIII core molecules. Factor VIIIa preparations contained heterotrimers (mass 162 +/- 13 kD) that had the same dimensions as fVIII core structures, lacked the B domainal appendage, and sometimes possessed the same core features as fVIII molecules. Molecular species corresponding to heterodimers (mass, 128 +/- 13 kD) and unassociated subunit chains (40-100 kD) were also observed in fVIIIa preparations, suggesting that heterotrimers have an appreciable tendency to dissociate, a phenomenon that could explain the decay of fVIIIa activity after thrombin activation of fVIII.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper H. A., Reisner F. F., Hall M., Wagner R. H. Effects of thrombin treatment of preparations of factor VIII and the Ca2+-dissociated small active fragment. J Clin Invest. 1975 Sep;56(3):751–760. doi: 10.1172/JCI108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. L., Wood W. I., Eaton D., Hass P. E., Hollingshead P., Wion K., Mather J., Lawn R. M., Vehar G. A., Gorman C. Construction and characterization of an active factor VIII variant lacking the central one-third of the molecule. Biochemistry. 1986 Dec 30;25(26):8343–8347. doi: 10.1021/bi00374a001. [DOI] [PubMed] [Google Scholar]
- Fass D. N., Knutson G. J., Katzmann J. A. Monoclonal antibodies to porcine factor VIII coagulant and their use in the isolation of active coagulant protein. Blood. 1982 Mar;59(3):594–600. [PubMed] [Google Scholar]
- Fay P. J., Anderson M. T., Chavin S. I., Marder V. J. The size of human factor VIII heterodimers and the effects produced by thrombin. Biochim Biophys Acta. 1986 Jun 23;871(3):268–278. doi: 10.1016/0167-4838(86)90208-6. [DOI] [PubMed] [Google Scholar]
- Fay P. J. Subunit structure of thrombin-activated human factor VIIIa. Biochim Biophys Acta. 1988 Jan 29;952(2):181–190. doi: 10.1016/0167-4838(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Fulcher C. A., Zimmerman T. S. Characterization of the human factor VIII procoagulant protein with a heterologous precipitating antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1648–1652. doi: 10.1073/pnas.79.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainfeld J. F., Wall J. S., Desmond E. J. A small computer system for micrograph analysis. Ultramicroscopy. 1982;8(3):263–270. doi: 10.1016/0304-3991(82)90242-x. [DOI] [PubMed] [Google Scholar]
- Hamer R. J., Koedam J. A., Beeser-Visser N. H., Sixma J. J. Human factor VIII: purification from commercial factor VIII concentrate, characterization, identification and radiolabeling. Biochim Biophys Acta. 1986 Oct 17;873(3):356–366. doi: 10.1016/0167-4838(86)90084-1. [DOI] [PubMed] [Google Scholar]
- Harmon J. T., Jamieson G. A., Rock G. A. The functional molecular weights of factor VIII activities in whole plasma as determined by electron irradiation. J Biol Chem. 1982 Dec 10;257(23):14245–14249. [PubMed] [Google Scholar]
- Hoyer L. W., Trabold N. C. The effect of thrombin on human factor VIII. Cleavage of the factor VIII procoagulant protein during activation. J Lab Clin Med. 1981 Jan;97(1):50–64. [PubMed] [Google Scholar]
- Hultin M. B., Jesty J. The activation and inactivation of human factor VIII by thrombin: effect of inhibitors of thrombin. Blood. 1981 Mar;57(3):476–482. [PubMed] [Google Scholar]
- Hultin M. B., Nemerson Y. Activation of factor X by factors IXa and VIII; a specific assay for factor IXa in the presence of thrombin-activated factor VIII. Blood. 1978 Nov;52(5):928–940. [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Dorner A. J. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988 May 5;263(13):6352–6362. [PubMed] [Google Scholar]
- Lollar P., Knutson G. J., Fass D. N. Stabilization of thrombin-activated porcine factor VIII:C by factor IXa phospholipid. Blood. 1984 Jun;63(6):1303–1308. [PubMed] [Google Scholar]
- Lollar P., Parker C. G. Stoichiometry of the porcine factor VIII-von Willebrand factor association. J Biol Chem. 1987 Dec 25;262(36):17572–17576. [PubMed] [Google Scholar]
- Lollar P., Parker C. G. Subunit structure of thrombin-activated porcine factor VIII. Biochemistry. 1989 Jan 24;28(2):666–674. doi: 10.1021/bi00428a038. [DOI] [PubMed] [Google Scholar]
- Lollar P., Parker C. G., Tracy R. P. Molecular characterization of commercial porcine factor VIII concentrate. Blood. 1988 Jan;71(1):137–143. [PubMed] [Google Scholar]
- Mosesson M. W., Hainfeld J., Wall J., Haschemeyer R. H. Identification and mass analysis of human fibrinogen molecules and their domains by scanning transmission electron microscopy. J Mol Biol. 1981 Dec 15;153(3):695–718. doi: 10.1016/0022-2836(81)90414-9. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Nesheim M. E., DiOrio J., Hainfeld J. F., Wall J. S., Mann K. G. Studies on the structure of bovine factor V by scanning transmission electron microscopy. Blood. 1985 May;65(5):1158–1162. [PubMed] [Google Scholar]
- Pittman D. D., Kaufman R. J. Proteolytic requirements for thrombin activation of anti-hemophilic factor (factor VIII). Proc Natl Acad Sci U S A. 1988 Apr;85(8):2429–2433. doi: 10.1073/pnas.85.8.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick M. E., Hoyer L. W. Thrombin activation of factor VIII: the effect of inhibitors. Br J Haematol. 1977 Aug;36(4):585–597. doi: 10.1111/j.1365-2141.1977.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Rotblat F., O'Brien D. P., O'Brien F. J., Goodall A. H., Tuddenham E. G. Purification of human factor VIII:C and its characterization by Western blotting using monoclonal antibodies. Biochemistry. 1985 Jul 30;24(16):4294–4300. doi: 10.1021/bi00337a007. [DOI] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Toole J. J., Pittman D. D., Orr E. C., Murtha P., Wasley L. C., Kaufman R. J. A large region (approximately equal to 95 kDa) of human factor VIII is dispensable for in vitro procoagulant activity. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5939–5942. doi: 10.1073/pnas.83.16.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Formation of a serine enzyme in the presence of bovine factor VIII (antihemophilic factor) and thrombin. Science. 1977 Jul 22;197(4301):374–376. doi: 10.1126/science.877560. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Keyt B., Eaton D., Rodriguez H., O'Brien D. P., Rotblat F., Oppermann H., Keck R., Wood W. I., Harkins R. N. Structure of human factor VIII. Nature. 1984 Nov 22;312(5992):337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]
- Wall J. S., Hainfeld J. F. Mass mapping with the scanning transmission electron microscope. Annu Rev Biophys Biophys Chem. 1986;15:355–376. doi: 10.1146/annurev.bb.15.060186.002035. [DOI] [PubMed] [Google Scholar]
- Weinstein M. J., Chute L. E. Two distinct forms of Factor VIII coagulant protein in human plasma. Cleavage by thrombin, and differences in coagulant activity and association with von Willebrand factor. J Clin Invest. 1984 Feb;73(2):307–316. doi: 10.1172/JCI111215. [DOI] [PMC free article] [PubMed] [Google Scholar]