Figure 3.
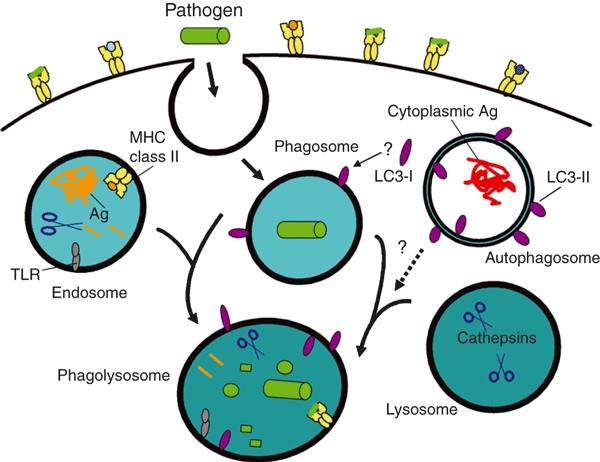
Autophagy, Atg proteins and pathogen phagocytosis. During phagocytosis, extracellular pathogens, such as bacteria, are internalized into phagosomes which fuse with endosomes and lysosomes to form phagolysosomes to promote pathogen degradation. Endosomes enriched for major histocompatibility complex (MHC) class II molecules, and select proteases, fuse with phagosomes, facilitating the association of antigenic peptides from the pathogen with class II molecules for presentation to CD4+ T cells. Disrupting the expression of Atg5, Atg7 or Atg16L1 proteins can perturb this pathway for MHC class II antigen (Ag) presentation. Lysosomes contribute digestive enzymes to the phagosome to promote its maturation into a phagolysosome. Signalling through toll-like receptor (TLR) or other innate receptors during phagocytosis can induce autophagy along with enhanced surface expression of MHC class II protein. Light chain-3 (LC3)-II is detected on phagolysosomes, yet there is currently no firm evidence to suggest that this is mediated by autophagosome fusion with phagosomes or phagolysosomes. Rather, LC3-I in the cytoplasm may be converted to LC3-II and sequestered onto phagocytic vesicles, along with other cytoplasmic cofactors that regulate organelle fusion and membrane remodelling. Alternatively, LC3-II may be delivered to endosomes (Fig. 1), which fuse with phagocytic organelles. Defining mechanistically how Atg proteins and autophagy pathways regulate phagocytosis and Ag cross-presentation remain important priorities.
