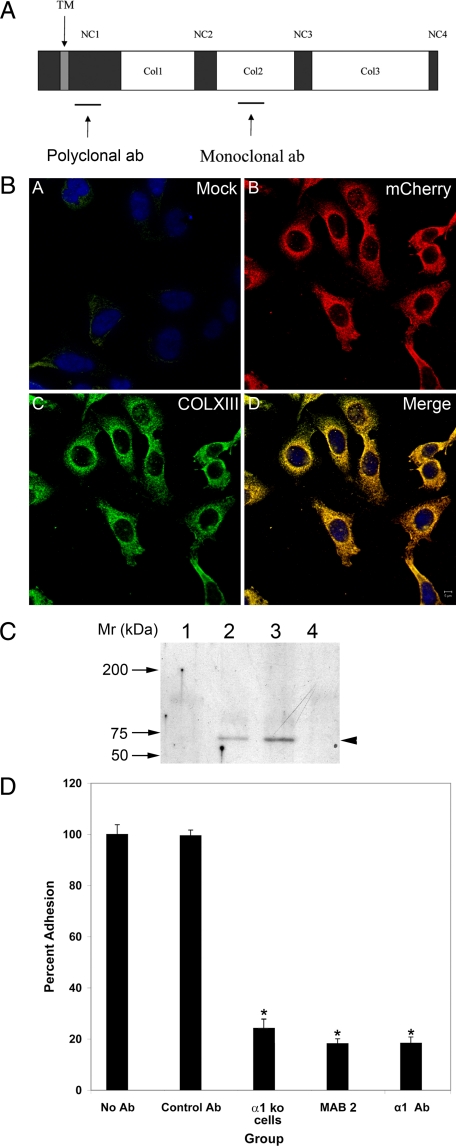
Figure 4.
Production and qualification of a mouse monoclonal antibody for the α1β1 integrin-binding site on collagen XIII. A: Cartoon of the structural components of trans-membrane collagen XIII, showing the location of the peptide immunogen used to raise monoclonal antibodies (based on sequence derived from phage display data), and polyclonal antibodies. B: CHO cells were either mock transfected (A) or transfected with a construct encoding a collagen XIII/mCherry chimeric protein (B, C, D). The cells were immunostained with Ab2 anti-collagen XIII mAb (A and C). mCherry (B) co-localizes with collagen XIII (D), demonstrating antibody specificity. C: Extracts from glomeruli (lane 1), Alport kidney endothelial cells (lane 2), and embryonic fibroblasts (lanes 3 and 4) were immunoprecipitated with Ab2 anti-collagen XIII antibodies (lanes 1–3) or IgM antibodies (lane 4). The immunoprecipitates were run on western blots and probed with rabbit polyclonal anti-collagen XIII antibodies. The arrowhead denotes a single band at the appropriate molecular weight for collagen XIII monomer. D: Monolayers of embryonic fibroblasts were used in cell adhesion experiments. Bone marrow-derived monocytes were applied to the monolayers in the presence or absence of either α1β1 integrin-neutralizing antibodies (α1 Ab), or monoclonal antibodies reactive to the peptide identified by biopanning as the binding site on collagen XIII for α1β1 integrin (MAB2). Bone marrow-derived monocytes from integrin α1 knockout mice were used as a control. The control antibody was an isotype matched (IgM) nonreactive monoclonal. Four independent experiments were performed in triplicate and analyzed statistically. There was significant differences in binding (*P > 0.05).