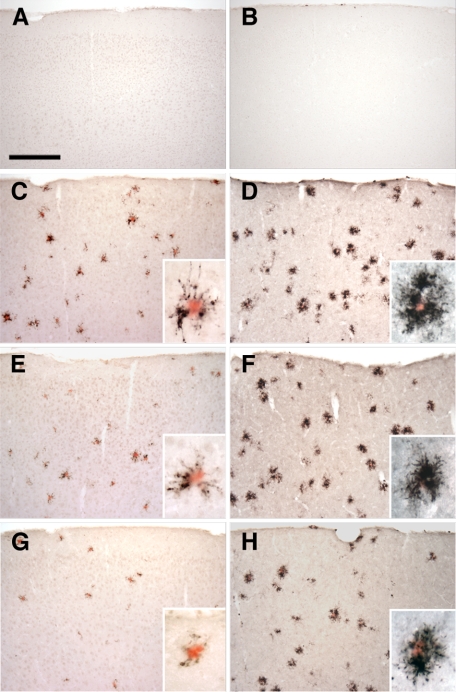
Figure 3.
Altered expression of microglial markers in the APPPS1 mouse model of AD with CX3CR1 deficiency. Brain sections (30 μm) from wild-type C57BL/6J (A and B), APPPS1;Cx3cr1+/+ (C and D), APPPS1;Cx3cr1+/− (E and F), and APPPS1;Cx3cr1−/− (G and H) mice at 4 months of age were immunostained with antibodies to either CD68, a marker for phagocytic microglia/macrophages (A, C, E, and G), or CD45, a marker for cells of hematopoietic lineage which is up-regulated in activated microglia (B, D, F, and H). Sections were also counterstained with Congo Red, a dye specific for dense-core Aβ deposits. Wild-type sections contained no Congo Red–positive deposits and rare CD68 (A) and CD45 (B) immunoreactivity. Consistent with the published literature, APPPS1;Cx3cr1+/+ animals show abundant CD68 (C) and CD45 immunoreactivity (D), especially in the immediate vicinity of Congo Red–positive Aβ deposits (insets). While age-matched APPPS1;Cx3cr1+/− and APPPS1;Cx3cr1−/− animals exhibit gene dose-dependent reduction in CD68 immunoreactivity compared to APPPS1;Cx3cr1+/+ controls (E and G, respectively), CD45 immunoreactivity is relatively unchanged in APPPS1;Cx3cr1+/− and APPPS1;Cx3cr1−/− animals compared with APPPS1;Cx3cr1+/+ controls (F and H, respectively). As expected, higher magnification revealed that CD68- and CD45-positive microglia are mostly clustered around Congo Red–positive Aβ deposits (insets). Scale bar = 250 μm.