Table 2.
Catalytic Enantioselective Synthesis of 3-Acyloxy-1-alkenesa
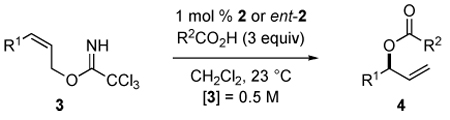 | |||||||
|---|---|---|---|---|---|---|---|
| Entrya | R1 | R2 | 3 | time [h] | 4 | yield [%]b | ee [%]c (config) |
| 1 | n-Pr | Me | 3b | 17 | 4b | 88 | 94 (R)d |
| 2e | n-Pr | Ph | 3b | 16 | 4b | 98 | 94 (R)f |
| 3 | i-Bu | Me | 3c | 14 | 4d | 96 | 93 |
| 4 | Cy | Me | 3d | 48 | 4h | 45 | 90 |
| 5 | (CH2)2Ph | Ph | 3e | 17 | 4i | 85 | 93g |
| 6 | CH2OH | Me | 3f | 17 | 4e | 92 | 97 (S)h |
| 7 | CH2OAc | Me | 3g | 8 | 4a | 90 | 99 (S)h |
| 8 | CH2OPMB | Me | 3h | 16 | 4f | 93 | 99 (S)h |
| 9 | (CH2)3OTBS | Me | 3i | 17 | 4g | 98 | 93 (R)i |
| 10j | (CH2)3OTBS | Ph | 3i | 20 | 4j | 93 | 99(S)d,g |
[(Rp,S)-COP-OAc]2 (2) except for entry 10 that used ent-2.
Yield of pure product.
Determined by GC analysis unless otherwise indicated; results of duplicate experiments agreed within ±2%.
Absolute configuration determined by analysis of the Mosher ester.34
[3] = 0.17 M.
Absolute configuration by optical rotation.35
Determined by HPLC analysis.
Absolute configuration by synthesis from (S)-3-butene-1,2-diol.
Absolute configuration by optical rotation.36
Catalyst was ent-2.
