Table 3.
Relative Reactivity of Carboxylic Acids with Allylic Imidate 3e in the Presence of 1 mol % of COP Catalyst 2a
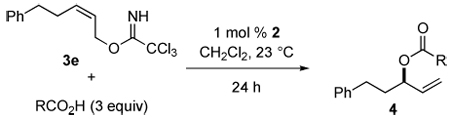 | ||||
|---|---|---|---|---|
| entry | R | pKa | % conversionb | |
| 10h | 24h | |||
| 1 | o-ClC6H4CH2 | 4.1 | 55 | 79 |
| 2 | Me | 4.8 | 65 | 89 |
| 3 | Ph | 4.2 | 62 | 84 |
| 4 | p-MeC6H4 | 4.4 | 42 | 67 |
| 5 | t-Bu | 5.0 | 24 | 40 |
| 6 | MeOCH2 | 3.6 | 13d | 38d |
| 7 | 1-adamantyl | 6.8 | 16 | 26 |
| 8 | ClCH2CH2 | 4.0 | 18c | 21 |
| 9 | o-ClC6H4 | 2.9 | 16d | 18d |
| 10 | Cl2CH | 1.3 | 18d | 18d |
3 Equiv RCO2H, 1 mol % 2, rt, [3e] = 0.5 M.
Determined by 1H NMR analysis of ester product 4 using an internal standard.
% Conversion at 3 h was 12%.
The major product of these reactions was the linear allylic acetate.
