Table 4.
Scope of the Enantioselective Reaction of Aromatic Carboxylic Acids with Imidate 3e to Form 3-Acyloxy-1-alkenes
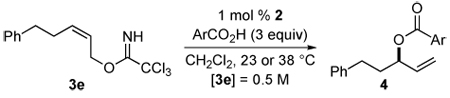 | ||||||
|---|---|---|---|---|---|---|
| entry | Ar | time [h] |
temp [°C] |
4 | yield [%]a |
ee [%]b |
| 1 | p-MeC6H4 | 17 | 23 | 4k | 85 | 93 |
| 2c | 2-naphthyl | 17 | 23 | 4l | 87 | 96 |
| 3 | p-MeOC6H4 | 22 | 38 | 4m | 79 | 86 |
| 4d | p-MeOC6H4 | 22 | 23 | 4m | 46 | 95 |
| 5 | p-PhC6H4 | 19 | 38 | 4n | 95 | 97e |
| 6 | p-ClC6H4 | 16 | 38 | 4o | 86 | 97 |
| 7 | p-NO2C6H4 | 22 | 38 | 4p | 65 | 87 |
| 8 | o-ClC6H4 | 18 | 38 | 4q | 24 | 46–53 |
Yield of pure product after silica gel chromatography.
Determined by HPLC analysis unless otherwise noted; results of duplicate experiments agreed within ±3%; absolute configuration assigned in analogy with products reported in Table 2.
3b used as substrate, [3b] = 0.17 M.
10:1 CH2Cl2:THF used as solvent.
Determined after conversion to benzoate ester 4i.
