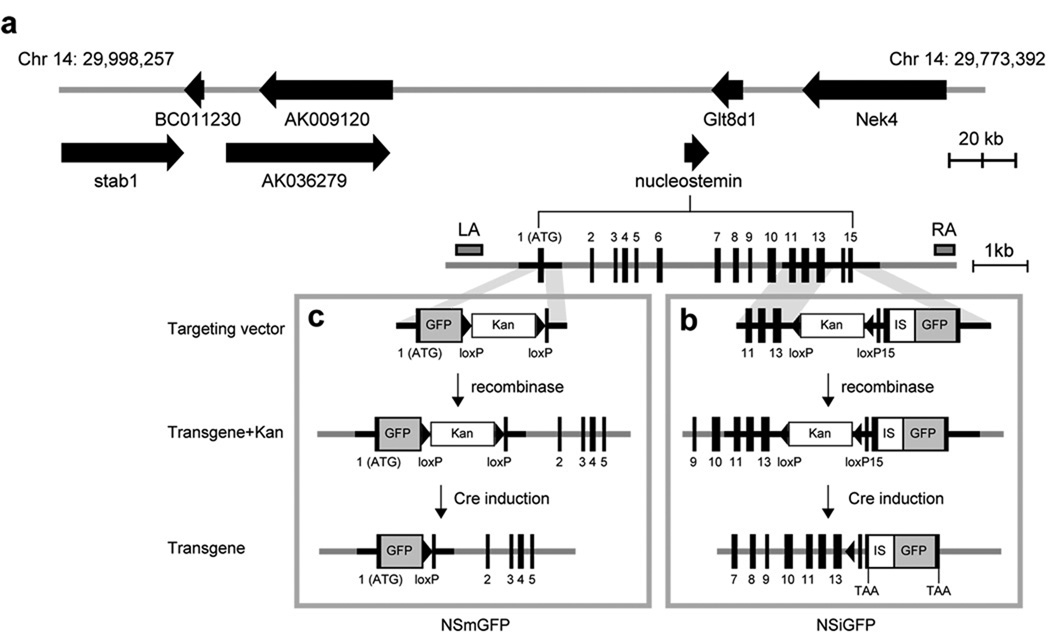
Figure 1. Generation of BAC transgenic mice using a bicistronic and an ATG-fusion strategy.
(a) A schematic diagram of the RP23-102M6 BAC (chromosome 14: 29,773,392-29,998,257). Arrows mark the coding regions and directions of known genes and hypothetical proteins. Accession numbers: stab1 (NM_138672, stabilin 1); AK009120 (polybromo-1 homologue); nucleostemin (NM_178846); Glt8d1 (NM_029626, glycosyltransferase 8 domain containing 1); Nek4 (AK149584, NIMA (never in mitosis gene a)-related expressed kinase 4). The nucleostemin locus contains 15 coding exons (black boxes). The positions of the left-arm (LA) and right-arm (RA) probes used in Fig. 2a are indicated. (b) In the bicistronic NSiGFP design, an IRES2 (IS)-GFP expression cassette was placed after the stop codon of nucleostemin. The targeting vector contained a loxP-flanked kanamycin selection cassette (Kan) to allow for clone selection in E.coli, a partial 3‘ sequence of nucleostemin before the stop codon, and the IS-GFP cassette, sandwiched by recombineering arms (shaded areas). After recombination in EL350 cells, the Kan cassette was excised by Cre recombinase, creating the NSiGFP transgene. (c) In the ATG-fusion NSmGFP design, GFP is expressed from the start codon of nucleostemin, thereby abolishing the expression of nucleostemin from the transgene.