Abstract
Background
Abnormal interactions between red blood cells, leukocytes and endothelial cells play a critical role in the occurrence of the painful vaso-occlusive crises associated with sickle cell disease. We investigated the interaction between circulating leukocytes and red blood cells which could lead to aggregate formation, enhancing the incidence of vaso-occlusive crises.
Design and Methods
Blood samples from patients with sickle cell disease (n=25) and healthy subjects (n=5) were analyzed by imaging and classical flow cytometry after density gradient separation. The identity of the cells in the peripheral blood mononuclear cell layer was determined using antibodies directed specifically against white (anti-CD45) or red (anti-glycophorin A) blood cells.
Results
Aggregates between red blood cells and peripheral blood mononuclear cells were visualized in whole blood from patients with sickle cell disease. The aggregation rate was 10-fold higher in these patients than in control subjects. Both mature red blood cells and reticulocytes were involved in these aggregates through their interaction with mononuclear cells, mainly with monocytes. The size of the aggregates was variable, with one mononuclear cell binding to one, two or several red blood cells. Erythroid Lu/basal cell adhesion molecule and α4β1 integrin were involved in aggregate formation. The aggregation rate was lower in patients treated with hydroxycarbamide than in untreated patients.
Conclusions
Our study gives visual evidence of the existence of circulating red blood cell-peripheral blood mononuclear cell aggregates in patients with sickle cell disease and shows that these aggregates are decreased during hydroxycarbamide treatment. Our results strongly suggest that erythroid Lu/basal cell adhesion molecule proteins are implicated in these aggregates through their interaction with α4β1 integrin on peripheral blood mononuclear cells.
Keywords: α4β1, Lu/BCAM, sickle cell disease, aggregates
Introduction
Sickle cell disease is a monogenic red blood cell disorder resulting from a single amino acid substitution in the hemoglobin (Hb) β-chain. This abnormal hemoglobin, named HbS, polymerizes under deoxygenated conditions leading to the formation of less deformable sickle red blood cells (SS RBC). Vaso-occlusive crises are the main acute complication of sickle cell disease and are the consequence of obstruction of blood microvessels by SS RBC.1 Indeed, in addition to their propensity to sickle, SS RBC can also adhere to vascular endothelium contributing to vaso-occlusive crises.2,3 Several studies, using in vitro and ex vivo models, have identified multiple adhesion proteins involved in SS RBC adhesion to endothelium. One of the most characterized RBC adhesion molecules is α4β1 integrin (or very late antigen-4, VLA-4), expressed on reticulocytes, which binds to vascular cell adhesion molecule-1 (VCAM-1), thrombospondin and fibronectin.4–7 Lutheran/basal cell adhesion molecule (Lu/BCAM) proteins, the unique receptors for laminin in normal (AA) and SS RBC,8–10 could be involved in vaso-occlusive crises. Unlike AA RBC, SS RBC adhere to laminin and resist high shear stress forces.8,11 Lu/BCAM-mediated SS RBC adhesion to laminin is stimulated by the physiological stress mediator epinephrine through the β2-adrenergic receptor and protein kinase A signaling pathway.12,13 Lu/BCAM proteins are also constitutively expressed on the endothelial cell surface and interact with α4β1 integrin expressed on young SS RBC, which may contribute to the abnormal adhesion of these RBC to resting endothelium.14
In addition to SS RBC, clinical observations suggested a role for leukocytes in the pathophysiological scheme of sickle cell disease.15–18 High leukocyte counts are associated with sickle cell disease-related morbidity and mortality19–23 and experimental studies suggested that leukocytes contribute to the vaso-occlusive process. Leukocytes from patients with sickle cell disease adhere abnormally to vascular endothelium in vitro24 and play a critical role in the vaso-occlusive phase of sickle cell retinopathy.25 Infusion of epinephrine-activated human SS RBC into nude mice induces vaso-occlusion associated with adhesion of murine leukocytes to vascular endothelium.26 Furthermore, sickle cell disease mice deficient in endothelial P and E-selectins, important mediators of leukocyte recruitment to the vessel wall, are protected from vaso-occlusion.27 Beside their adhesion to the vessel wall through interactions with endothelial and extracellular matrix proteins, leukocytes can also interact with circulating SS RBC leading to the formation of aggregates and enhancing the incidence of vaso-occlusive crises. SS RBC adhere to polymorphonuclear neutrophils in vitro28 and are captured by adherent leukocytes, as shown by intravital microscopy in mice expressing human HbS27 as well as in a flow model of vaso-occlusion in vitro.29 Brittain et al. showed interactions between reticulocytes and monocytes in whole blood samples and in adhesion assays in vitro.30 These interactions are mediated by α4β1 integrin, expressed on both cell types, via a bridge of soluble fibronectin.30 Furthermore, in vitro experiments suggested that SS RBC bind to peripheral blood mononuclear cells (PBMC) via erythroid LW/ICAM-4 and CD44 receptors, and induce their adhesion to endothelium.31
In this study we used innovative imaging flow cytometry technique to visualize directly RBC-PBMC aggregates in a layer of enriched PBMC obtained by density gradient separation of SS whole blood. We studied the nature of these aggregates, the protein interactions facilitating their formation and the effects of hydroxycarbamide treatment, given that this drug is know to reduce the frequency of vaso-occlusive crises.32
Design and Methods
Patients
Homozygous sickle cell disease patients (SS) at least 18 years old, able to give their informed consent and consulting our Adult Sickle-Cell Referral Center were eligible for inclusion in this study which was approved by the local ethics committee (Comité de Protection des Personnes) and was conducted in accordance with the provisions of the Declaration of Helsinki, and local laws and regulations. Experiments were performed with freshly drawn heparin-anticoagulated venous blood from healthy adult donors or adult SS patients at steady-state, treated or not with hydroxycarbamide. A patient was defined as having steady state disease when at least 1 month had passed since an acute clinical event and at least 3 months since a blood transfusion. We selected 17 SS patients untreated with hydroxycarbamide (mean age: 34 years, SD: 8 years; male to female sex ratio: 12/5) and eight patients on stable hydroxycarbamide therapy (20 mg/kg/day for >6 months) (mean age: 37 years, SD: 10 years; male to female sex ratio: 4/4). These two groups were studied independently. Blood samples from five healthy adult donors (AA) were used as control samples.
Preparation of the peripheral blood mononuclear cell layer
Venous blood was collected from the antecubital vein into lithium heparin (17 U.l/mL) tubes (Becton Dickinson Vacutainer, Plymounth, UK). PBMC were separated from whole blood using Ficoll-Histopaque-1077 (Sigma-Aldrich, Saint Louis, MO, USA) density gradient separation and the isolated cells were washed once with phosphate-buffered saline containing calcium (1 mM) and magnesium (1 mM).
Labeling and blocking experiments
Cells were incubated with mouse monoclonal anti-human CD45-fluorescein isothiocyanate (FITC) and glycophorin A (GPA)-phycoerythrin (PE) or GPA-allophycocycanin (APC) conjugated antibodies (Becton Dickinson Biosciences, San Jose, CA, USA) for 60 min and washed with phosphate-buffered saline before flow cytometry or imaging flow cytometry analysis. For triple staining experiments, monoclonal anti-human CD71-APC conjugated antibody (Becton Dickinson Biosciences) was added. Indirect labeling was used for Lu/BCAM: cells were incubated with a goat poly-clonal antibody anti-human Lu/BCAM (R&D systems, Minneapolis, MN, USA) for 90 min, washed with phosphate-buffered saline, then incubated with a swine anti-goat-PE secondary antibody (Beckman Coulter, Fullerton, CA, USA) together with anti-CD45-FITC and anti-GPA-APC antibodies.
For blocking experiments, the reagents were added prior to labeling. Cells from the mononuclear enriched layer were incubated for 30 min with 40 μg/mL recombinant human VCAM-1-Fc chimera (R&D systems, Minneapolis, MN, USA) or 40 μg/mL mouse monoclonal anti-human CD29 antibody (Becton Dickinson Biosciences).
Flow cytometry analysis
Acquisition and analysis were performed using a BD FACS Canto II flow cytometer (Becton Dickinson Biosciences) via BD FACSDiva software (v6.1.2). A total of 5×105 events were collected for each experiment. Monocytes and lymphocytes of the PBMC layer were identified and gated by forward and side scatter. PBMC, RBC and aggregates were quantified as the percentage of events that stained positive for CD45 (% CD45+), GPA (% GPA+) and both antigens (% CD45+ GPA+), respectively. The percentage of PBMC involved in aggregates among total CD45+ events (% PBMC in aggregates) was determined for each subject according to the following formula:
Imaging flow cytometry analysis
Acquisition and analysis were performed using the ImageStream system (Amnis Corporation, Seattle, WA, USA) and the ImageStream Data Exploration and Analysis Software (IDEAS; Amnis). This software enables quantitative characterization of single cells or aggregates within a population by assessing a combination of morphology and fluorescence patterns. The ImageStream system is equipped with three laser lines 405, 488 and 658 nm. Samples were prepared as described above. Spectral compensation was performed as described by Ortyn et al.33 Cell populations were identified by gating on cells expressing surface markers and confirmed by visual inspection of the fluorescence pattern.
Statistical analyses
The Mann-Whitney test was used to determine: (i) differences in RBC and PBMC proportions between untreated SS patients and AA subjects; (ii) differences in the percentage of PBMC in aggregates between untreated SS patients and AA subjects and between untreated and hydroxycarbamide-treated SS patients. Blocking experiments were analyzed using Wilcoxon's matched-pairs test. P values less than 0.05 were considered statistically significant.
Results
Abnormal co-selection of red blood cells and peripheral blood mononuclear cells in patients with sickle cell disease
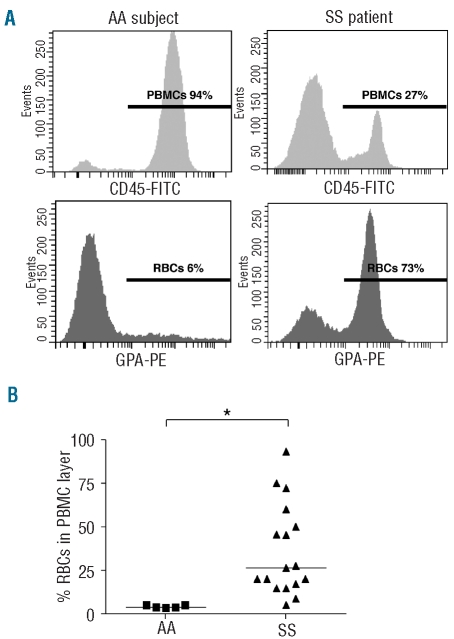
PBMC were isolated from whole blood samples by Ficoll-Histopaque gradient separation. Cells from the PBMC layer were analyzed by flow cytometry using FITC-conjugated anti-CD45 and PE-conjugated anti-GPA antibodies specific for white blood cells and RBC, respectively. As illustrated in Figure 1A, an abnormally high percentage of RBC, determined by the percentage of GPA-PE events, was observed in the PBMC layer of SS patients (typical result, n=17). This percentage was highly variable among SS patients and the median percentage was significantly higher for SS patients than for AA controls (26.4 versus 3.7, P=0.0013) (Figure 1B).
Figure 1.
Abnormal SS RBC co-selection during PBMC density gradient separation. (A) Typical histograms representing flow cytometry analysis of the PBMC layer in one AA subject (left panels) and one SS patient (right panels). Upper and lower panels represent CD45-FITC (PBMC) and GPA-PE (RBC) staining, respectively. The horizontal lines represent areas of positive events; percentages indicate the proportions of positive events for each marker. (B) RBC percentage in the PBMC layer in AA subjects (▪) (n=5) and SS patients (▴) (n=17). Horizontal lines indicate medians. The percentage of RBC in the PBMC layer is significantly higher in SS patients than in AA subjects. *P=0.0013, Mann-Whitney test.
High rate of red blood cell and peripheral blood mononuclear cell aggregates in patients with sickle cell disease
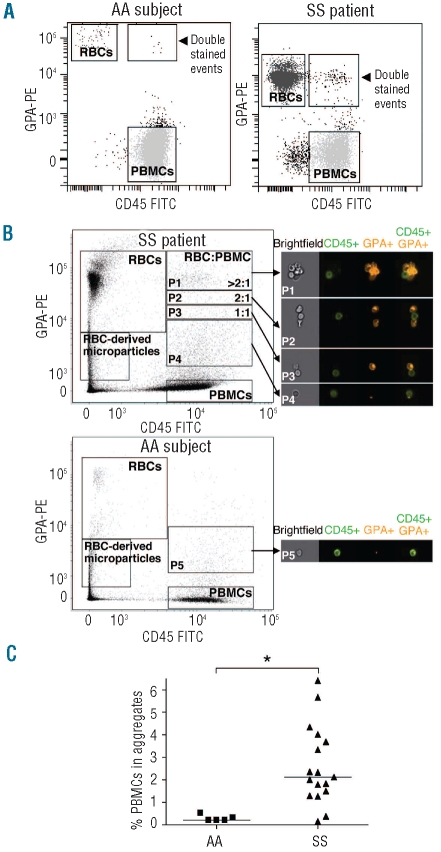
A high number of events double-positive for both CD45-FITC and GPA-PE were detected in all SS patients in contrast to AA subjects (Figure 2A, typical result, n=17). We postulated that these events could represent aggregates between RBC and PBMC. To test this hypothesis, we used imaging flow cytometry. We analyzed the PBMC layer of one SS patient and one AA subject after CD45-FITC and GPA-PE staining. Images obtained from the SS patient showed that the double-stained events did indeed correspond to PBMC-RBC aggregates (Figure 2B). Three populations of PBMC-RBC aggregates could be distinguished according to the GPA-PE intensity, all of which involved one PBMC which interacted with either one, two, or more than two RBC. A fourth population involving PBMC and small RBC-derived microparticles was also observed. The rare double-stained events observed in AA subjects were mainly PBMC interacting with RBC-derived microparticles (Figure 2B, AA subject).
Figure 2.
SS patients have abnormally high rates of RBC-PBMC aggregates. (A) Typical dot plot representation of the PBMC layer flow cytometry analysis in one AA subject (left panel) and one SS patient (right panel). CD45-FITC and GPA-PE antibodies stain PBMC and RBC, respectively. CD45-FITC and GPA-PE double-stained events represent potential RBC-PBMC aggregates. (B) Imaging flow cytometry showing dot plots of one SS patient (upper left panel) and one AA subject (lower left panel), as in (A). Right panels show typical examples of brightfield and fluorescent images from the area of double-stained events. Fluorescent images show, from left to right, CD45-FITC-labeled PBMC, GPA-PE-labeled RBC or RBC-derived microparticles and CD45-FITC/GPA-PE double-stained events. The double-stained area was divided into four parts according to the RBC:PBMC ratio in aggregates: >2:1 (P1), 2:1 (P2), 1:1 (P3). The fourth part shows the presence of PBMC interacting with RBC-derived microparticles (P4). Double-stained events from the AA subject were essentially restricted to PBMC interacting with RBC-derived microparticles (P5). (C) Percentages of PBMC involved in aggregates in AA subjects (▪) (n=5) and SS patients (▴) (n = 17). Horizontal lines indicate medians. *P=0.0048, Mann-Whitney test.
Based on the imaging flow cytometry findings, gating of double-stained events was improved in classical flow cytometry experiments, excluding the aggregates between PBMC and RBC-derived microparticles. As the RBC percentage in the PBMC layer was variable among SS patients, the aggregation rate was determined by calculating the percentage of PBMC involved in the aggregates. The median of this percentage was 10-fold higher in SS patients than in healthy controls (2.11 versus 0.2, P=0.0048) (Figure 2C).
Aggregates include reticulocytes, mature red blood cells and monocytes
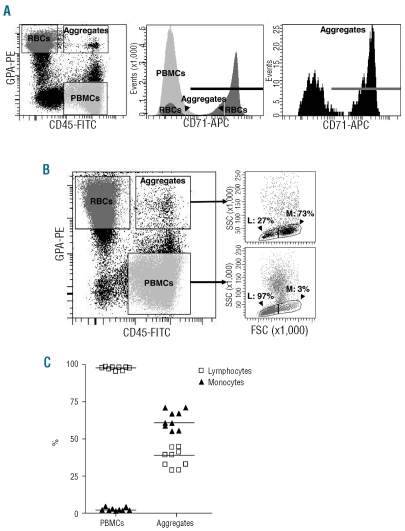
To determine the maturity of the SS RBC population involved in aggregation, cells were double stained with anti-GPA and anti-CD71 antibody which recognizes the transferrin receptor, specific to reticulocytes among the RBC population. First, the RBC population of the PBMC layer (Figure 3A, typical result, left panel) was analyzed and showed the presence of both mature RBC and reticulocytes (Figure 3A, middle panel). The percentage of reticulocytes in this layer differed among the seven tested patients but was always higher than that in whole blood, indicating a preferential selection of reticulocytes during the density separation. When aggregates were gated, they showed CD71-positive and CD71-negative populations, indicating that both young and mature RBC were involved in aggregation with PBMC (Figure 3A, right panel). The median of CD71-positive RBC in aggregates was 58.4% (n=7).
Figure 3.
SS RBC and PBMC populations involved in aggregation. (A) Aggregates include both mature SS RBC and reticulocytes. Typical results showing cells from the PBMC layer labeled with FITC-conjugated anti-CD45, PE-conjugated anti-GPA and APC-conjugated anti-CD71 antibodies. The dot plot representation in the left panel shows gated events corresponding to SS RBC, PBMC and aggregates. Each of these three populations was gated and analyzed for CD71 expression, as shown in the middle panel. CD71 expression in aggregates is magnified in the right panel. The bold horizontal lines represent CD71-positive events. (B) Monocytes have a greater ability to aggregate than lymphocytes. The dot plot representation in the left panel is as for (A). Lymphocytes (L) and monocytes (M) were distinguished among PBMC and aggregates using morphological parameters (forward versus side scatter, right panels). (C) Percentage of lymphocytes (□) and monocytes (▴) in the PBMC population and in aggregates. Horizontal lines indicate medians.
The percentages of lymphocytes and monocytes in the PBMC population and in the aggregates of nine patients were determined using forward and side scatters (Figure 3B, typical result). In the PBMC population, the majority of the cells were lymphocytes (median: 97.8%). Despite the small percentage of monocytes in the PBMC population (median: 2.2%), they represented the majority of PBMC involved in aggregation (median: 60.7%), indicating that RBC preferentially interacted with monocytes within the aggregates (Figure 3C).
α4β1 integrin and erythroid Lu/basal cell adhesion molecule are involved in aggregate formation
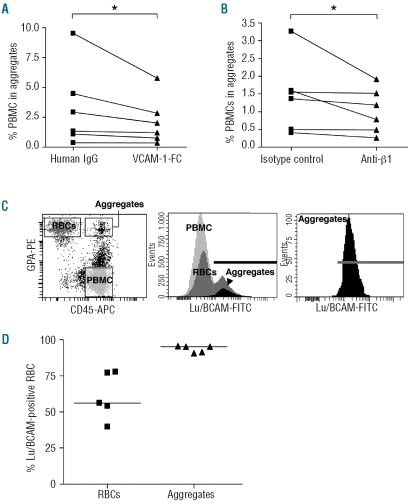
To identify the adhesion proteins involved in aggregate formation, inhibition assays were performed with specific ligands of α4β1 integrin, which is expressed on all PBMC and a population of reticulocytes. When soluble VCAM-1-Fc was added, the percentage of aggregates was significantly reduced (median: 2.4% versus 3.7%, n=6, P=0.03) (Figure 4A). Incubation with anti-β1 blocking antibody also inhibited aggregation in six other samples (median: 0.98% versus 1.45%, P=0.03) (Figure 4B), indicating that α4β1 integrin was involved in the formation of the aggregates.
Figure 4.
α4β1 integrin and Lu/BCAM are involved in RBC-PBMC aggregation. Inhibition assays using VCAM-1-Fc (A) or anti-β1 antibody (B). *P=0.03, Wilcoxon's matched-pairs test. (C) The dot plot representation in the left panel shows gated events corresponding to SS RBC, PBMC and aggregates. Each of these three populations was gated and analyzed for Lu/BCAM expression, as shown in the middle panel. Lu/BCAM expression in aggregates is magnified in the right panel. Bold horizontal lines represent Lu/BCAM-positive events. (D) Percentage of Lu/BCAM-positive events in the RBC population (▴) and in aggregates (▾) of five SS patients. Horizontal lines indicate medians.
As Lu/BCAM glycoproteins are known to interact with α4β1 integrin,14 their implication in aggregation was investigated. Lu/BCAM antigens vary greatly in strength among individuals and exhibit heterogeneity between individual RBC within a person. This accounts for the mixed field agglutination patterns showing clumps of agglutinated cells in the presence of free cells.34 The distribution of Lu/BCAM antigens among the PBMC layer cells was analyzed. As expected, Lu/BCAM were not expressed on PBMC35 (Figure 4C, middle panel). Heterogeneous expression of Lu/BCAM on RBC within the same individual was confirmed for all sickle cell disease patients analyzed in this experiment (Figure 4C, middle panel, typical result). The patients showed different proportions of Lu/BCAM-negative and -positive (median: 56.1%, n=5) RBC in the PBMC layer (Figure 4D). The heterogeneity of Lu/BCAM distribution found among total RBC was not conserved in the aggregates as all RBC involved in aggregates stained positive for Lu/BCAM (median: 95.1%) (Figure 4C, right panel, 4D). The absence of Lu/BCAM-negative RBC and the exclusive presence of Lu/BCAM-positive RBC in aggregates strongly suggested that Lu/BCAM were the erythroid proteins involved in the PBMC-RBC interaction.
Aggregation rate is lower in patients treated with hydroxycarbamide
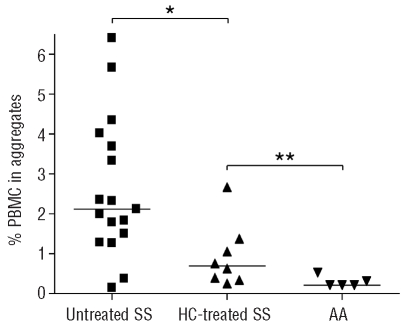
The effect of hydroxycarbamide treatment on RBC-PBMC aggregation was investigated. Eight hydroxycarbamide-treated patients were analyzed and compared to 17 untreated patients. The median of the aggregation percentage was significantly lower for the treated patients than for the untreated ones (0.68% versus 2.11%, P=0.013) (Figure 5), indicating that hydroxycarbamide diminished the occurrence of aggregation. However, more aggregates were still detected in blood samples from hydroxycarbamide-treated patients than in those from healthy controls (median: 0.68% versus 0.29%, P=0.0186) (Figure 5).
Figure 5.
Hydroxycarbamide (HC) decreases the rate of RBC-PBMC aggregation in SS patients. Percentage of PBMC in aggregates in SS untreated patients (▪, n=17), HC-treated patients (▴, n=8) and AA subjects (▾, n=5). *P=0.013, **P=0.0186, Mann-Whitney test.
Discussion
Leukocytes are believed to play a critical role in sickle cell disease by aggregating with RBC and platelets and by adhering to and stimulating the vascular endothelium. This stimulation leads to increased expression of RBC adhesion molecule ligands, thus contributing to vaso-occlusion.15 In the present study, we revealed the presence of aggregates involving SS RBC and PBMC in whole blood from sickle cell disease patients after enrichment of PBMC by density gradient separation. Previous studies using in vitro binding assays,28,30 a sickle cell disease mouse model27 and an in vitro flow model of vaso-occlusion29 suggested interactions between the two cell types in sickle cell disease.
Because of their size and their multiple potential interactions with the vascular wall, leukocyte-RBC aggregates could initiate or aggravate vaso-occlusion by disrupting microcirculatory blood flow. Intravital microscopy studies in a sickle cell disease mouse model revealed that adherent leukocytes in inflamed venules played a direct role in vaso-occlusion by trapping circulating SS RBC.27 Inhibition of leukocyte-RBC interactions with a high dose of intra-venous immune globulin prevented venular vaso-occlusion in these mice.36 A novel mechanism that may contribute to vaso-occlusion was described, in which incubating leukocytes with SS RBC, in particular with epinephrine-activated SS RBC, stimulated their adhesion to endothelial cells in vitro.31 Our imaging flow cytometry analysis gave strong evidence of the abnormal RBC-PBMC interactions that could occur in sickle cell disease patients’ whole blood, as circulating leukocyte-RBC aggregates were visualized for the first time. Based on the study by Zennadi et al.,31 the presence of such interactions in whole blood could lead to activation of PBMC and contribute to abnormal cell adhesion to the vascular wall. The imaging flow cytometry revealed that a PBMC could interact with more than one SS RBC in cell aggregates. It also enabled the visualization of aggregates between PBMC and RBC-derived microparticles in both sickle cell disease patients and AA controls. The imaging flow cytometry assay was essential to determine the composition of the aggregates and to optimize the gating in the classical flow cytometry assays that followed, taking into account aggregates comprising whole cells and excluding those with RBC-derived microparticles.
Our study indicated that both SS reticulocytes and mature RBC were involved in aggregation with PBMC. In contrast, Brittain et al. reported that reticulocytes represented the primary cells interacting with leukocytes.30 Our results were obtained after specific cell surface labeling of reticulocytes and showed the presence of both cell types (reticulocytes and mature RBC) in cell aggregates, whereas Brittain et al. showed a positive correlation between the number of GPA+/CD45+ events and reticulocyte count, which does not exclude the presence of mature RBC in aggregates. Our finding is supported by the results of a study by Zennadi et al. in which both SS reticulocytes and mature RBC were shown to interact with PBMC and induce their adhesion to endothelial cells in vitro.31 Moreover, in prior studies, dense (mostly mature) SS RBC were more adherent to polymorphonuclear neutrophil monolayers than light (mostly young) SS RBC,28 reinforcing our finding concerning the ability of mature SS RBC to interact with leukocytes.
In our study, SS RBC interacted preferentially with monocytes within the aggregates, as previously described in whole blood.30 These interactions could result from the presence of activated monocytes, as sickle cell disease is characterized by an inflammatory state reflected by activation of monocytes37–39 and polymorphonuclear neutrophils.40 Although the majority of the PBMC involved in aggregates were monocytes, 39.33% (median) were lymphocytes, indicating the ability of these latter cells to interact with SS RBC. Zennadi et al. reported that SS RBC induced adhesion of both lymphocytes and monocytes to endothelial cells,31 suggesting an interaction between SS RBC and both PBMC types. Our results strongly support the presence of direct interactions between lymphocytes and SS RBC in vivo, suggesting that, in addition to neutrophils and monocytes, lymphocytes may also contribute to the pathophysiology of sickle cell disease.
We identified α4β1 integrin and Lu/BCAM as cell surface proteins involved in RBC-PBMC aggregation. α4β1 integrin is expressed on leukocytes and reticulocytes whereas Lu/BCAM expression is specific to the erythroid lineage among circulating blood cells. The involvement of α4β1 in RBC-PBMC aggregation was shown by the inhibition assays using VCAM-1-Fc and anti-β1 blocking antibody. Inhibition was variable but statistically significant in two sets of six patients, even though the aggregation was not totally abolished. This was probably due to the difficulty of VCAM-1-Fc and anti-β1 antibody to compete with the established interaction between α4β1 and its erythroid lig-and. An interaction between reticulocyte α4β1 and endothelial Lu/BCAM had already been described in sickle cell disease patients.14 Our current study suggested that α4β1 on the surface of PBMC could interact with erythroid Lu/BCAM in the RBC-PBMC aggregates. Plasma fibronectin was found to mediate interactions between monocytes and SS reticulocytes by bridging α4β1 molecules on both sides in in vitro adhesion assays and in blood from patients with sickle cell disease.30 Our experiments showed that both SS reticulocytes and mature RBC, which do not express α4β1, were involved in aggregates, suggesting that cell-cell interactions were not dependent on fibronectin. It has also been shown that epinephrine-treated SS RBC induce PBMC adhesion to endothelial cells via at least two erythroid receptors, LW and CD44.31 Nevertheless, the Lu/BCAM-α4β1 interaction seems to be the primary interaction responsible for the RBC-PBMC aggregates that we identified, since no Lu/BCAM-negative RBC were found within these aggregates.
We also investigated the effect of hydroxycarbamide treatment on RBC-PBMC aggregation. Hydroxycarbamide therapy is associated with clinical benefits in sickle cell disease by reducing the frequencies of painful vaso-occlusive crises and admissions to hospital32 but its mechanism of action is still poorly understood. We found that the rates of aggregates were lower in hydroxycarbamide-treated patients than in untreated patients. A similar observation was made in a flow model of vaso-occlusion in which hydroxycarbamide inhibited the interactions between adherent leukocytes and flowing SS RBC in vitro.29 The inhibition that we detected was probably not due to a lower expression of Lu/BCAM on the surface of SS RBC, as hydroxycarbamide-treatment increases the percentage of Lu/BCAM-positive SS RBC and the level of Lu/BCAM expression/RBC.41 It could be due to the inhibition of Lu/BCAM and/or α4β1 activities as both molecules are known to be activated by phosphorylation of their cytoplasmic domain.6,13 Hydroxycarbamide therapy for sickle cell disease also targets leukocytes by reducing their number, decreasing their activation and modulating their expression of adhesion molecules.42–45 Although hydroxycarbamide decreases α4β1 expression on SS reticulocytes,41,46 to our knowledge, the effect of hydroxycarbamide-treatment on α4β1 in sickle cell disease leukocytes has not been investigated yet. A recent study showed that hydroxycarbamide treatment diminishes the expression of genes encoding for inflammatory mediators, such as tumor necrosis factor-α, in sickle cell disease mononuclear cells.44 Such a decrease in tumor necrosis factor-α could have a direct inhibitory effect on sickle cell disease mononuclear cell activation and could thus modulate α4β1 binding activity.47 The decrease of RBC-PBMC aggregation associated with hydroxycarbamide treatment could explain, in part, the clinical benefit of hydroxycarbamide therapy: more investigations are necessary to confirm this hypothesis.
In conclusion, our study provided visual evidence of the existence of RBC-PBMC aggregates in patients with sickle cell disease and showed that the aggregation rate is decreased during hydroxycarbamide treatment. Our results strongly suggest that erythroid Lu/BCAM are involved in this aggregation through their interaction with α4β1 integrin on PBMC. Future investigations should help to characterize the mechanisms leading to this interaction and evaluate the impact of aggregates on the occurrence of vaso-occlusive crises, which could generate new therapeutic perspectives.
Acknowledgment
the authors would like to thank Marie-Paule Wautier for helpful advice. Funding: this investigation was supported by the Institut National de la Transfusion Sanguine (INTS), the Institut National de la Santé et de la Recherche Médicale (INSERM), Université Paris Diderot Paris 7, a grant from the Agence Nationale de la Recherche (ANR, SCADHESION 2007), two grants from Région Ile-de-France (SESAME 2007 no. F-08-1104/R and IMAGO-POLE Project 2007) and a “maladies rares” fellowship from the Société Française de Médecine Interne (SNFMI) and Actélion.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Embury SH, Hebbel RP, Mohandas N, Steinberg MH. Pathogenesis of vasoocclusion. In: Embury SH, Hebbel RP, Mohandas N, Steinberg MH, editors. Sickle Cell Disease: Basic Principles and Clinical Practice. New York, NY: Raven Press; 1994. pp. 311–26. [Google Scholar]
- 2.Hoover R, Rubin R, Wise G, Warren R. Adhesion of normal and sickle erythrocytes to endothelial monolayer cultures. Blood. 1979;54(4):872–6. [PubMed] [Google Scholar]
- 3.Hebbel RP, Yamada O, Moldow CF, Jacob HS, White JG, Eaton JW. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980;65(1):154–60. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gee BE, Platt OS. Sickle reticulocytes adhere to VCAM-1. Blood. 1995;85(1):268–74. [PubMed] [Google Scholar]
- 5.Joneckis CC, Ackley RL, Orringer EP, Wayner EA, Parise LV. Integrin alpha 4 beta 1 and glycoprotein IV (CD36) are expressed on circulating reticulocytes in sickle cell anemia. Blood. 1993;82(12):3548–55. [PubMed] [Google Scholar]
- 6.Brittain JE, Han J, Ataga KI, Orringer EP, Parise LV. Mechanism of CD47-induced alpha4beta1 integrin activation and adhesion in sickle reticulocytes. J Biol Chem. 2004;279(41):42393–402. doi: 10.1074/jbc.M407631200. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Eckmam JR, Swerlick RA, Wick TM. Phorbol ester stimulation increases sickle erythrocyte adherence to endothelium: a novel pathway involving alpha 4 beta 1 integrin receptors on sickle reticulocytes and fibronectin. Blood. 1996;88(11):4348–58. [PubMed] [Google Scholar]
- 8.Udani M, Zen Q, Cottman M, Leonard N, Jefferson S, Daymont C, et al. Basal cell adhesion molecule/lutheran protein. The receptor critical for sickle cell adhesion to laminin. J Clin Invest. 1998;101(11):2550–8. doi: 10.1172/JCI1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Nemer W, Gane P, Colin Y, Bony V, Rahuel C, Galacteros F, et al. The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J Biol Chem. 1998;273(27):16686–93. doi: 10.1074/jbc.273.27.16686. [DOI] [PubMed] [Google Scholar]
- 10.Parsons SF, Lee G, Spring FA, Willig TN, Peters LL, Gimm JA, et al. Lutheran blood group glycoprotein and its newly characterized mouse homologue specifically bind alpha5 chain-containing human laminin with high affinity. Blood. 2001;97(1):312–20. doi: 10.1182/blood.v97.1.312. [DOI] [PubMed] [Google Scholar]
- 11.Hillery CA, Du MC, Montgomery RR, Scott JP. Increased adhesion of erythrocytes to components of the extracellular matrix: isolation and characterization of a red blood cell lipid that binds thrombospondin and laminin. Blood. 1996;87(11):4879–86. [PubMed] [Google Scholar]
- 12.Hines PC, Zen Q, Burney SN, Shea DA, Ataga KI, Orringer EP, et al. Novel epinephrine and cyclic AMP-mediated activation of BCAM/Lu-dependent sickle (SS) RBC adhesion. Blood. 2003;101(8):3281–7. doi: 10.1182/blood-2001-12-0289. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier E, Rahuel C, Wautier MP, El Nemer W, Gane P, Wautier JL, et al. Protein kinase A-dependent phosphorylation of Lutheran/basal cell adhesion molecule glycoprotein regulates cell adhesion to laminin alpha5. J Biol Chem. 2005;280(34):30055–62. doi: 10.1074/jbc.M503293200. [DOI] [PubMed] [Google Scholar]
- 14.El Nemer W, Wautier MP, Rahuel C, Gane P, Hermand P, Galacteros F, et al. Endothelial Lu/BCAM glycoproteins are novel ligands for red blood cell alpha4beta1 integrin: role in adhesion of sickle red blood cells to endothelial cells. Blood. 2007;109 (8):3544–51. doi: 10.1182/blood-2006-07-035139. [DOI] [PubMed] [Google Scholar]
- 15.Okpala I. The intriguing contribution of white blood cells to sickle cell disease - a red cell disorder. Blood Rev. 2004;18(1):65–73. doi: 10.1016/s0268-960x(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 16.Okpala I. Leukocyte adhesion and the pathophysiology of sickle cell disease. Curr Opin Hematol. 2006;13(1):40–4. doi: 10.1097/01.moh.0000190108.62414.06. [DOI] [PubMed] [Google Scholar]
- 17.Abboud M, Laver J, Blau CA. Granulocytosis causing sickle-cell crisis. Lancet. 1998;351(9107):959. doi: 10.1016/S0140-6736(05)60614-9. [DOI] [PubMed] [Google Scholar]
- 18.Adler BK, Salzman DE, Carabasi MH, Vaughan WP, Reddy VV, Prchal JT. Fatal sickle cell crisis after granulocyte colony-stimulating factor administration. Blood. 2001;97(10):3313–4. doi: 10.1182/blood.v97.10.3313. [DOI] [PubMed] [Google Scholar]
- 19.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 20.Castro O, Brambilla DJ, Thorington B, Reindorf CA, Scott RB, Gillette P, et al. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84(2):643–9. [PubMed] [Google Scholar]
- 21.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–94. [PubMed] [Google Scholar]
- 22.Powars DR. Management of cerebral vasculopathy in children with sickle cell anaemia. Br J Haematol. 2000;108(4):666–78. doi: 10.1046/j.1365-2141.2000.01912.x. [DOI] [PubMed] [Google Scholar]
- 23.Kinney TR, Sleeper LA, Wang WC, Zimmerman RA, Pegelow CH, Ohene-Frempong K, et al. Silent cerebral infarcts in sickle cell anemia: a risk factor analysis. The Cooperative Study of Sickle Cell Disease. Pediatrics. 1999;103(3):640–5. doi: 10.1542/peds.103.3.640. [DOI] [PubMed] [Google Scholar]
- 24.Fadlon E, Vordermeier S, Pearson TC, Mire-Sluis AR, Dumonde DC, Phillips J, et al. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91(1):266–74. [PubMed] [Google Scholar]
- 25.Kunz Mathews M, McLeod DS, Merges C, Cao J, Lutty GA. Neutrophils and leucocyte adhesion molecules in sickle cell retinopathy. Br J Ophthalmol. 2002;86(6):684–90. doi: 10.1136/bjo.86.6.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zennadi R, Moeller BJ, Whalen EJ, Batchvarova M, Xu K, Shan S, et al. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood. 2007;110(7):2708–17. doi: 10.1182/blood-2006-11-056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci USA. 2002;99(5):3047–51. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofstra TC, Kalra VK, Meiselman HJ, Coates TD. Sickle erythrocytes adhere to polymorphonuclear neutrophils and activate the neutrophil respiratory burst. Blood. 1996;87(10):4440–7. [PubMed] [Google Scholar]
- 29.Finnegan EM, Turhan A, Golan DE, Barabino GA. Adherent leukocytes capture sickle erythrocytes in an in vitro flow model of vaso-occlusion. Am J Hematol. 2007;82(4):266–75. doi: 10.1002/ajh.20819. [DOI] [PubMed] [Google Scholar]
- 30.Brittain JE, Knoll CM, Ataga KI, Orringer EP, Parise LV. Fibronectin bridges monocytes and reticulocytes via integrin alpha4beta1. Br J Haematol. 2008;141(6):872–81. doi: 10.1111/j.1365-2141.2008.07056.x. [DOI] [PubMed] [Google Scholar]
- 31.Zennadi R, Chien A, Xu K, Batchvarova M, Telen MJ. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood. 2008;112(8):3474–83. doi: 10.1182/blood-2008-01-134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–22. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 33.Ortyn WE, Hall BE, George TC, Frost K, Basiji DA, Perry DJ, et al. Sensitivity measurement and compensation in spectral imaging. Cytometry A. 2006;69(8):852–62. doi: 10.1002/cyto.a.20306. [DOI] [PubMed] [Google Scholar]
- 34.Daniels G. Lutheran Blood Group System. In: Daniels G, editor. Human Blood Groups. Oxford, UK: Blackwell Science; 1995. pp. 356–84. [Google Scholar]
- 35.Rahuel C, Le Van Kim C, Mattei MG, Cartron JP, Colin Y. A unique gene encodes spliceoforms of the B-cell adhesion molecule cell surface glycoprotein of epithelial cancer and of the Lutheran blood group glycoprotein. Blood. 1996;88(5):1865–72. [PubMed] [Google Scholar]
- 36.Turhan A, Jenab P, Bruhns P, Ravetch JV, Coller BS, Frenette PS. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood. 2004;103(6):2397–400. doi: 10.1182/blood-2003-07-2209. [DOI] [PubMed] [Google Scholar]
- 37.Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96(7):2451–9. [PubMed] [Google Scholar]
- 38.Wun T, Cordoba M, Rangaswami A, Cheung AW, Paglieroni T. Activated monocytes and platelet-monocyte aggregates in patients with sickle cell disease. Clin Lab Haematol. 2002;24(2):81–8. doi: 10.1046/j.1365-2257.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 39.Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102(7):2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 40.Lard LR, Mul FP, de Haas M, Roos D, Duits AJ. Neutrophil activation in sickle cell disease. J Leukoc Biol. 1999;66(3):411–5. doi: 10.1002/jlb.66.3.411. [DOI] [PubMed] [Google Scholar]
- 41.Odievre MH, Bony V, Benkerrou M, Lapoumeroulie C, Alberti C, Ducrocq R, et al. Modulation of erythroid adhesion receptor expression by hydroxyurea in children with sickle cell disease. Haematologica. 2008;93(4):502–10. doi: 10.3324/haematol.12070. [DOI] [PubMed] [Google Scholar]
- 42.Saleh AW, Hillen HF, Duits AJ. Levels of endothelial, neutrophil and platelet-specific factors in sickle cell anemia patients during hydroxyurea therapy. Acta Haematol. 1999;102(1):31–7. doi: 10.1159/000040964. [DOI] [PubMed] [Google Scholar]
- 43.Benkerrou M, Delarche C, Brahimi L, Fay M, Vilmer E, Elion J, et al. Hydroxyurea corrects the dysregulated L-selectin expression and increased H(2)O(2) production of poly-morphonuclear neutrophils from patients with sickle cell anemia. Blood. 2002;99(7):2297–303. doi: 10.1182/blood.v99.7.2297. [DOI] [PubMed] [Google Scholar]
- 44.Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leukoc Biol. 2009;85(2):235–42. doi: 10.1189/jlb.0708445. [DOI] [PubMed] [Google Scholar]
- 45.Okpala I, Daniel Y, Haynes R, Odoemene D, Goldman J. Relationship between the clinical manifestations of sickle cell disease and the expression of adhesion molecules on white blood cells. Eur J Haematol. 2002;69(3):135–44. doi: 10.1034/j.1600-0609.2002.02775.x. [DOI] [PubMed] [Google Scholar]
- 46.Styles LA, Lubin B, Vichinsky E, Lawrence S, Hua M, Test S, et al. Decrease of very late activation antigen-4 and CD36 on reticulocytes in sickle cell patients treated with hydroxyurea. Blood. 1997;89(7):2554–9. [PubMed] [Google Scholar]
- 47.Shimizu Y, Van Seventer GA, Horgan KJ, Shaw S. Regulated expression and binding of three VLA (beta 1) integrin receptors on T cells. Nature. 1990;345(6272):250–3. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]