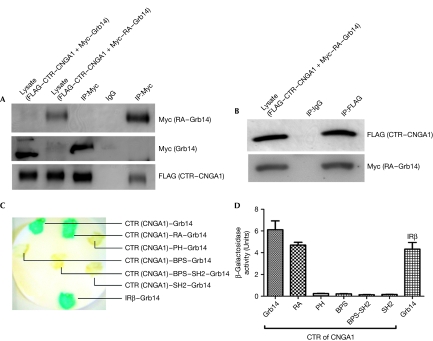
Figure 5.
Carboxy-terminal region of cyclic nucleotide-gated channel alpha subunit interacts with the Ras-associating domain of growth factor receptor-bound protein 14. (A) RA domain of Grb14 or full-length Grb14 were co-expressed with CTR–CNGA1 in HEK293T cells. Protein extracts expressing Myc–RA–Grb14 or Myc–Grb14 were co-immunoprecipitated with Myc antibody. The presence of CTR–CNGA1 in the immunoprecipitate was tested by immunoblot analysis using FLAG antibody and the blot was reprobed with Myc antibody for RA–Grb14 and Grb14. (B) Protein extracts expressing CTR–CNGA1 and RA–Grb14 were subjected to reciprocal co-immunoprecipitation with FLAG antibody. The presence of RA–Grb14 in the immunoprecipitate was tested by immunoblot analysis using Myc antibody and the blot was reprobed with FLAG antibody for CTR–CNGA1. (C) The L40 yeast strain was transformed with pLexA–CTR–CNGA1 and Grb14 or various individual domains of Grb14 and analysed by yeast two-hybrid assay. Positive control included the co-expression of IRβ and Grb14. Transformants were assayed for β-galactosidase by colony colour by filter lift assay and (D) the β-galactosidase activity was quantitatively determined by the solution assay. cGMP, cyclic guanosine monophosphate; CNGA1, cyclic nucleotide-gated channel alpha subunit; CTR, C-terminal region; Grb14, growth factor receptor-bound protein 14; IR, insulin receptor β-subunit; PH, Pleckstrin homology; RA, Ras-associating.