Abstract
Cardiovascular disease (CVD) is the leading cause of death in renal allograft recipients with functioning graft. Our study aimed to determine the incidence and the risk factors of cardiovascular disease after renal transplantation in Korea. We retrospectively analyzed 430 adult recipients who underwent kidney transplantation between January 1997 and February 2007. CVD was defined as a composite outcome of ischemic heart disease, cerebrovascular accident and peripheral vascular disease. Mean age of recipients was 40.0±11.8 yr. Mean duration of follow-up was 72±39 months. The cumulative incidence of CVD after renal transplantation was 2.4% at 5 yr, 5.4% at 10 yr and 11.4% at 12 yr. Multivariate analysis revealed that recipient's age, diabetes mellitus and duration of dialysis before transplantation were associated with post-transplant CVD (hazard ratio 1.843 [95% CI, 1.005-3.381], 3.846 [95% CI, 1.025-14.432] and 3.394 [95% CI, 1.728-6.665] respectively). In conclusion, old age, duration of dialysis and diabetes mellitus are important risk factors for post-transplant CVD, although the incidence of post-renal transplant CVD is lower in Korea than that in western countries.
Keywords: Cardiovascular Diseases, Incidence, Kidney Transplantation, Risk Factors, Diabetes Mellitus, Koreans
INTRODUCTION
Kidney transplantation is now one of the standard treatment options of end stage renal disease patients, which can give opportunities even for highly sensitized patients (1, 2). Cardiovascular disease is the leading cause of death in renal allograft recipients with functioning graft (3). There were approximately 6- and 10-fold higher incidences in adult allograft recipients, comparing that of the general population (4). In a recent report, cardiovascular disease, including angina pectoris, cerebrovascular accident (CVA) and peripheral vascular disease (PVD), occurred in 25% of the patients at 10 yr and 53% of the patients at 15 yr after renal transplantation (5). Another study based on the United States Renal Data System (USRDS), myocardial infarction (MI) after kidney transplantation occurred in 11.1% of patients by 3 yr post-transplantation. However, there was no report about the incidence of cardiovascular disease after renal transplantation in Asian population, although ethnic disparity in the incidence of cardiovascular disease exists (6).
Renal allograft recipients have a higher prevalence of traditional Framingham atherosclerotic vascular disease risk factors (7). In addition to traditional risk factors, variables related to end stage renal disease (ESRD), allograft function, donor and immunosuppression are also important risk factors for cardiovascular disease (4, 5, 8, 9). Although there was a recent attempt to find clinical risk factors to the progression of coronary atherosclerosis score (10), significance of risk factors of cardiovascular disease, either traditional or nontraditional, has not been evaluated in Asian renal allograft recipients. Our study aimed to determine the incidence and the risk factors of cardiovascular disease after renal transplantation in Korea.
MATERIALS AND METHODS
Subjects
Medical records of 554 patients who underwent renal transplantation between January 1996 and February 2007 at Seoul National University Hospital were reviewed retrospectively. Data of 124 patients were excluded from analysis for various reasons: age younger than 18 yr (n=94), combined organ transplantation (n=15, 13 for simultaneous pancreas-kidney co-transplantation, one for liver-kidney co-transplantation, and one for heart-kidney co-transplantation) and unavailable medical records (n=14). Finally, 430 patients were included in the study. Institutional review board of Seoul National University Hospital approved this study (IRB No:H-1002-001-307). The study is conducted under the Declaration of Helsinki.
Definitions
Cardiovascular disease was defined as a composite outcome of ischemic heart disease (IHD), CVA and PVD. The diagnosis of IHD included angina pectoris, coronary artery revascularization (percutaneous coronary intervention or coronary artery bypass grafting), MI, or death attributable to IHD. Angina pectoris was accepted when it was verified by medical records with objective evidence (abnormal coronary angiography or myocardial scintigraphy). MI was diagnosed by a history of typical chest pain and myocardial enzyme elevation. CVA was defined as a composite outcome of ischemic stroke or documented transient ischemic attack. Intracerebral hemorrhage (ICH) was excluded. PVD was limited to lesions that needed revascularization. In the case of diabetic foot, only amputation was accepted as PVD.
Clinical variables
Variables recorded at the time of transplantation included: age, gender, weight, height, body mass index (BMI, kg/m2), cause of ESRD, modality of renal replacement therapy (RRT), duration of RRT, donor type (deceased/living), donor age, donor gender, number of HLA mismatches, cigarette smoking history, diabetes mellitus, hypertension, dyslipidemia, previous cardiovascular diseases (IHD, CVA or PVD), baseline echocardiographic finding and type of initial calcineurin inhibitor (cyclosporine A vs FK-506).
Post-transplant variables were as follows: the development of post-transplant diabetes mellitus (PTDM) and glomerular filtration rate (GFR) at one year determined by using simplified Modification of Diet in Renal Disease (MDRD) study equation.
Statistical analysis
Continuous variables were expressed as arithmetic mean±SD. Chi-square test (or Fisher's exact test) and independent t-test were carried out to compare the characteristics of population between patients with cardiovascular disease and patients without, as appropriate. Univariate Cox regression analysis was performed to find risk factors of cardiovascular disease. Risk factors with a P value of <0.20 were kept to be included in multivariate analysis. Multivariate analysis was performed using Cox regression models. Variables that showed a missing rate of over 10% were not accepted into the multivariate analysis model. Kaplan-Meier survival curves were also generated. The survival curves between diabetes patients and non-diabetes patients were compared by Log-rank test. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using the SPSS statistics package version 12.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
Baseline characteristics
In 430 patients, mean age was 40±11.8 yr and 63% were men. Mean body mass index was 22±3.0 kg/m2. The most common cause of ESRD was glomerulonephritis (31.4%), followed by hypertension (12.8%), diabetes (7.2%), and autosomal dominant polycystic kidney disease (ADPKD) (2.3%). Patients had a history of smoking or were still smoking at the time of transplantation in 23.4%. Most of patients (91.2%) were hypertensive before transplantation. Diabetes mellitus was prevalent in 9.8% of patients. Mean duration of diabetes mellitus was 13.4±8.3 yr. PTDM occurred in 15.7% of patients. Most PTDM (67%) occurred within three months after renal transplantation. PTDM was more prevalent in patients who used FK-506, but it did not show statistical significance (19.5% vs 12.9%, P=0.092). Patients previously diagnosed as IHD and CVA were 3.8% and 3.7%, respectively.
The majority of patients were transplanted with a living donor kidney (84.4%). Mean donor age was 37±11.2 yr. Sixteen percent of patients underwent kidney transplantation without dialysis (preemptive transplantation). In patients who had received dialysis, the mean duration of RRT was 30.9±42.48 months. All patients received calcineurin inhibitor-based immunosuppression (FK-506 vs cyclosporine A, 43.1%:56.9%, as initial calcineurin inhibitor).
Incidence of cardiovascular disease after renal transplantation
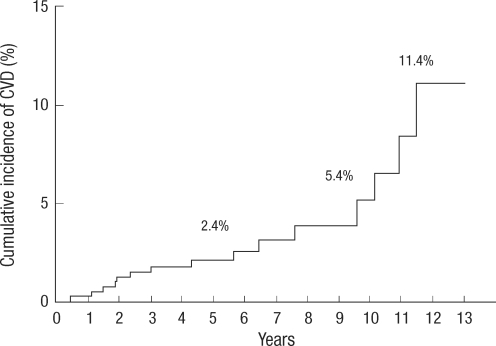
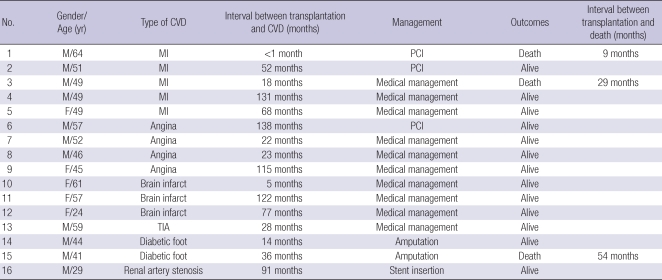
CVD occurred in 16 patients during follow up. The mean duration of follow up was 72±39 months (range, 0-156 months). Fig. 1 shows the cumulative incidence of CVD after renal transplantation: 2.4% at 5 yr, 5.4% at 10 yr, and 11.4% at 12 yr. Table 1 describes detailed information of CVD. CVD occurred in various time periods (range 0-131 months). MI occurred in five patients and two of them died. The other mortality occurred in a PVD patient due to an infection.
Fig. 1.
The cumulative incidence of cardiovascular disease after renal transplantation. The estimated percentage equals 2.4% after 5 yr, 5.4% after 10 yr and 11.4% after 12 yr.
Table 1.
Clinical characteristics of cardiovascular disease after renal transplantation
CVD, cardiovascular disease; MI, myocardial infarct; PCI, Percutaneous coronary intervention; TIA, transient ischemic attack.
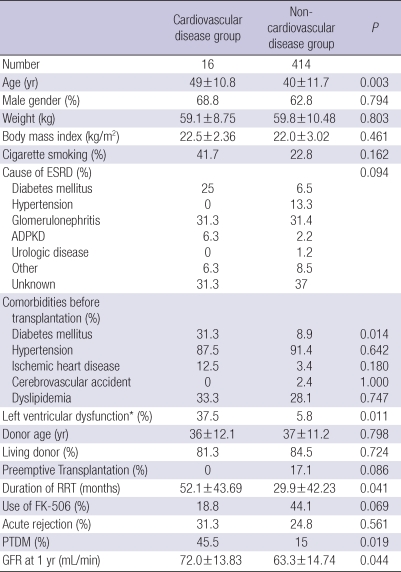
Table 2 summarizes the differences of baseline clinical characteristics between CVD (n=16) and non-CVD (n=414) groups. Mean age of CVD group was about 10 yr higher than that of non-CVD group (49±10.8 vs 40±11.7 yr, P=0.003). Diabetes was more prevalent in CVD group (31.3% vs 8.9%, P=0.014). More patients in CVD group had left ventricular dysfunction (defined by an ejection fraction less than 45% on echocardiography, 37.5% vs 5.8%, P=0.011). The duration of RRT was longer in CVD group (52.1±43.69 vs 29.9±42.23 months, P=0.041). However, other variables such as gender proportion, weight, BMI, history of smoking, cause of ESRD, donor age, donor type, preemptive transplantation, type of calcineurin inhibitor and acute rejection indicated no significant difference.
Table 2.
Comparison of baseline characteristics of renal allograft recipients
Data are expressed as mean±SD or percentage.
*Left ventricular dysfunction was defined as ejection fraction <45%.
ESRD, end stage renal disease; ADPKD, autosomal dominant polycystic kidney disease; PTDM, post-transplantation diabetes mellitus; CsA, cyclosporine A; RRT, renal replacement therapy; GFR, glomerular filtration rate.
Risk factors for cardiovascular disease
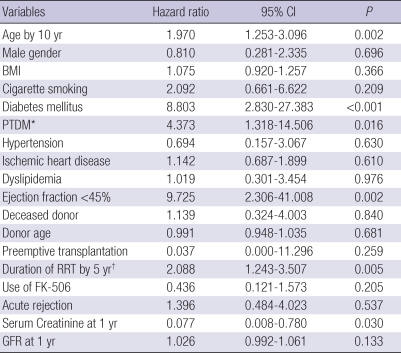
Table 3 gives a result of univariate Cox regression analysis. In univariate analysis, older age (hazard ratio [HR] 1.970, 95% confidence interval [CI] 1.253-3.096, P=0.001), presence of diabetes mellitus (HR 8.803, 95% CI 2.830-27.383, P<0.001), occurrence of PTDM (HR 4.373, 95% CI 1.318-14.506, P=0.016), left ventricular dysfunction (HR 9.725, 95% CI 2.306-41.008, P=0.002) and longer duration of RRT (HR 2.088, 95% CI 1.243-3.507, P=0.005) were significantly associated with CVD. In contrast, there was no association with recipient's gender, BMI, cigarette smoking, hypertension before transplantation, IHD before transplantation and dyslipidemia. Among non-traditional risk factors, donor type, donor age, preemptive transplantation, use of FK-506 and acute rejection showed no significant association with CVD.
Table 3.
Univariate analysis of risk factors predicting cardiovascular disease after renal transplantation
*Patients with diabetes mellitus before transplantation were excluded; †Preemptive transplantation was considered as zero month of RRT duration.
CI, Confidence interval; BMI, body mass index; PTDM, post-transplantation diabetes mellitus; RRT, renal replacement therapy; GFR, glomerular filtration rate.
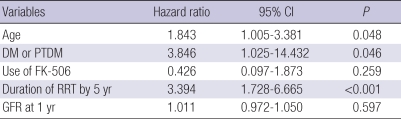
Table 4 shows a result of multivariate analysis. LV dysfunction and cigarette smoking were excluded from multivariate risk model because of missing data rates. Age (HR 1.843 P=0.048), duration of renal replacement therapy (HR 3.394, P<0.001) and either presence of diabetes mellitus or occurrence of PTDM (HR 3.846, P=0.046) were independent risk factors of cardiovascular disease after renal transplantation.
Table 4.
Multivariate analysis of risk factors predicting cardiovascular disease after renal transplantation
CI, Confidence interval; DM, diabetes mellitus; PTDM, post-transplantation diabetes mellitus; RRT, renal replacement therapy; GFR, glomerular filtration rate.
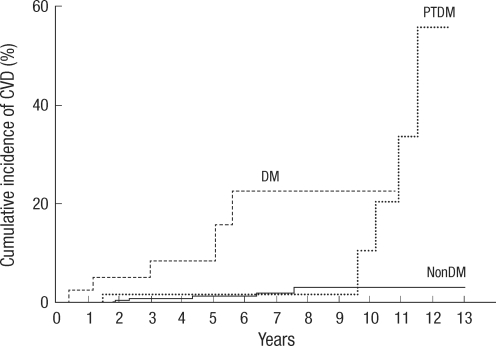
Fig. 2 is a Kaplan-Meier curve which depicts occurrence of cardiovascular disease according to diabetes status (diabetes before transplantation group, PTDM group and non-diabetes group). In Fig. 2, CVD tends to occur in the later period in PTDM group. Median time interval from transplantation to CVD was 122 months in PTDM group. (Diabetes vs PTDM vs non-diabetes, 14 months: 122 months: 40 months, P<0.001) In addition, CVD also tends to occur in the later period in PTDM group compared with diabetes mellitus group (P=0.025).
Fig. 2.
Differences in the cumulative incidence of cardiovascular disease according to diabetes status. Median time interval from transplantation to CVD was significantly different between groups. (Diabetes vs PTDM vs non-diabetes, 14 months: 122 months: 40 months, P<0.001, Log rank test).
DISCUSSION
Our study shows the significantly lower incidence of CVD than previous reports: the cumulative incidence of CVD was 11.4% at 12 yr after transplantation in our cohort. This result could be attributed to the lower prevalence of diabetes mellitus. The prevalence of diabetes in a previous report from the United States was 44.7%. Our study population included 9.8% of diabetes patients, nearly one fifth of USRDS data (4). In addition, the low incidence might be related to ethnic disparity. A prevalent risk factor itself may not be sufficient to predict a high CVD incidence in Asian population. For example, in a study to compare the observed coronary heart disease rate with predicted coronary heart disease rate in Chinese population, the observed coronary heart disease rate was only one sixth of predicted rate calculated by Framingham function (11).
For renal transplantation recipients, diabetes seems to have special importance. In a corner stone study that compared the CVD risk factors of renal transplant recipients to that of the Framingham Heart Study (FHS) population, risk of IHD associated with diabetes mellitus was substantially higher for renal transplant recipients than for the FHS population. It was postulated that an advanced form of diabetes mellitus (e.g., longer duration of diabetes mellitus, poor glycemic control and unknown genetic susceptibility of microvasculature) may have been more prevalent among renal transplant recipients than among diabetic patients in the FHS cohort, because diabetes mellitus in renal transplant recipients had already caused ESRD (12, 13). PTDM has been reported to be associated with cardiovascular risk in renal recipients (14, 15). Our study also proved PTDM as a CVD risk factor. Furthermore, in our study, CVD tended to occur in later periods in PTDM group, which suggests a possibility that the cumulative effect of hyperglycemia on the occurrence of CVD. Since PTDM is a common comorbidity after renal transplantation, about 10% of prevalence (16), it is worth investigating prospectively whether optimal management of PTDM reduces CVD occurrence.
Left ventricular dysfunction was a significant risk factor in univariate analysis, although it was omitted from multivariate analysis because missing rates were too high (27.3% of missing rates). However, it may have some clinical significance. Since neuropathy is common in diabetes or ESRD patients, asymptomatic IHD could occur. A low ejection fraction detected by echocardiography could reflect the sequelae of silent IHD. A longer duration of RRT before transplantation was associated with a higher incidence of CVD, which is consistent with the previous report (17). It was suggested that there is an association of long term dialysis with the inflammatory biomarker and vascular calcification (18, 19). However, it is the limitation of this study that there were no additional biochemical data to link the duration of RRT to the risk of CVD. Preemptive transplantation was negatively correlated with CVD occurrence in univariate analysis. Several previous studies included dialysis duration as covariates and two studies reported lower CVD occurrence in preemptive transplantation group in univariate analysis (4, 5). No previous study proved the preemptive transplantation as an independent negative risk factor. Neither hypertension nor dyslipidemia was a significant risk factor in our study. By definition, all patients who used anti-hypertensive drugs or statins were regarded as hypertensive patients or dyslipidemic patients. The very high prevalence of hypertension irrespective of CVD and use of active medications might reduce the significance of these comorbidities as risk factors.
FK-506 has been reported to improve cardiovascular risk profile (9, 20, 21). At the same time, FK-506 has been demonstrated to increase the rate of occurrence of PTDM. Although an improved cardiovascular risk profile has been shown in several reports, only a single study showed that the use of FK-506 actually reduces the observed incidence of CVD (22). We performed subgroup analysis to establish cardiovascular risk of FK-506 in non-diabetic patients. However, it did not demonstrate the difference (data not shown). One plausible explanation is that the occurrence of PTDM could offset the beneficial effect to lipid profile in FK-506 treated patients. It should be established by a long term prospective study.
In summary, this is the first study about the incidence of CVD and its risk factors in Korean renal transplant recipients: the cumulative incidence of CVD after renal transplantation is 11.4% at 12 yr. Independent predictive factors associated with the occurrence of CVD include advanced age, prolonged duration of RRT and either presence of diabetes mellitus or occurrence of PTDM.
References
- 1.Kim SM, Lee C, Lee JP, Kim EM, Ha J, Kim SJ, Park MH, Ahn C, Kim YS. Kidney transplantation in sensitized recipients; a single center experience. J Korean Med Sci. 2009;24(Suppl):S143–S147. doi: 10.3346/jkms.2009.24.S1.S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon HE, Hyoung BJ, Hwang HS, Lee SY, Jeon YJ, Song JC, Oh EJ, Park SC, Choi BS, Moon IS, Kim YS, Yang CW. Successful renal transplantation with desensitization in highly sensitized patients: a single center experience. J Korean Med Sci. 2009;24(Suppl):S148–S155. doi: 10.3346/jkms.2009.24.S1.S148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.USRDS 2008 Annual report. USRDS. [accssed on 28 September 2009]. Available at http://www.usrds.org/2008/pdf/V2_07_2008.pdf.
- 4.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16:496–506. doi: 10.1681/ASN.2004070580. [DOI] [PubMed] [Google Scholar]
- 5.Vanrenterghem YF, Claes K, Montagnino G, Fieuws S, Maes B, Villa M, Ponticelli C. Risk factors for cardiovascular events after successful renal transplantation. Transplantation. 2008;85:209–216. doi: 10.1097/TP.0b013e318160254f. [DOI] [PubMed] [Google Scholar]
- 6.Harland JO, Unwin N, Bhopal RS, White M, Watson B, Laker M, Alberti KG. Low levels of cardiovascular risk factors and coronary heart disease in a UK Chinese population. J Epidemiol Community Health. 1997;51:636–642. doi: 10.1136/jech.51.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT., Jr Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 8.Kasiske BL, Maclean JR, Snyder JJ. Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol. 2006;17:900–907. doi: 10.1681/ASN.2005090984. [DOI] [PubMed] [Google Scholar]
- 9.Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, Blom HJ, Sweep FC, Demacker PN, Hilbrands LB. Improved cardiovascular risk profile and renal function in renal transplant patients after randomized conversion from cyclosporine to tacrolimus. J Am Soc Nephrol. 2003;14:1880–1888. doi: 10.1097/01.asn.0000071515.27754.67. [DOI] [PubMed] [Google Scholar]
- 10.Kim HW, Kang SW, Lee HY, Choi DH, Shim WH, Kim SI, Kim YS, Choi KH. Correlates of the severity of coronary atherosclerosis in long-term kidney transplant patients. J Korean Med Sci. 2010;25:706–711. doi: 10.3346/jkms.2010.25.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Hong Y, D'Agostino RB, Sr, Wu Z, Wang W, Sun J, Wilson PW, Kannel WB, Zhao D. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA. 2004;291:2591–2599. doi: 10.1001/jama.291.21.2591. [DOI] [PubMed] [Google Scholar]
- 12.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 13.Kasiske BL, Chakkera HA, Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11:1735–1743. doi: 10.1681/ASN.V1191735. [DOI] [PubMed] [Google Scholar]
- 14.Cosio FG, Kudva Y, van der Velde M, Larson TS, Textor SC, Griffin MD, Stegall MD. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415–2421. doi: 10.1111/j.1523-1755.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 15.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 16.Shah T, Kasravi A, Huang E, Hayashi R, Young B, Cho YW, Bunnapradist S. Risk factors for development of new-onset diabetes mellitus after kidney transplantation. Transplantation. 2006;82:1673–1676. doi: 10.1097/01.tp.0000250756.66348.9a. [DOI] [PubMed] [Google Scholar]
- 17.Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM, Leichtman AB, Kaplan B. Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58:1311–1317. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 18.Mallamaci F, Tripepi G, Cutrupi S, Malatino LS, Zoccali C. Prognostic value of combined use of biomarkers of inflammation, endothelial dysfunction, and myocardiopathy in patients with ESRD. Kidney Int. 2005;67:2330–2337. doi: 10.1111/j.1523-1755.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez D, Rufino M, Bartolomei S, Gonzalez-Rinne A, Lorenzo V, Cobo M, Torres A. Clinical impact of preexisting vascular calcifications on mortality after renal transplantation. Kidney Int. 2005;67:2015–2020. doi: 10.1111/j.1523-1755.2005.00303.x. [DOI] [PubMed] [Google Scholar]
- 20.Ligtenberg G, Hene RJ, Blankestijn PJ, Koomans HA. Cardiovascular risk factors in renal transplant patients: cyclosporin A versus tacrolimus. J Am Soc Nephrol. 2001;12:368–373. doi: 10.1681/ASN.V122368. [DOI] [PubMed] [Google Scholar]
- 21.Morales JM, Dominguez-Gil B. Impact of tacrolimus and mycophenolate mofetil combination on cardiovascular risk profile after kidney transplantation. J Am Soc Nephrol. 2006;17:S296–S303. doi: 10.1681/ASN.2006080930. [DOI] [PubMed] [Google Scholar]
- 22.Shihab FS, Waid TH, Conti DJ, Yang H, Holman MJ, Mulloy LC, Henning AK, Holman J, Jr, First MR. Conversion from cyclosporine to tacrolimus in patients at risk for chronic renal allograft failure: 60-month results of the CRAF Study. Transplantation. 2008;85:1261–1269. doi: 10.1097/TP.0b013e31816b4388. [DOI] [PubMed] [Google Scholar]