Abstract
A 50-yr-old male presented a thyroid mass with dysphasia and hoarseness. He underwent total thyroidectomy and neck node dissection. Pathologically, the tumor had two distinct tumor components with intermingled areas: follicular variant of papillary carcinoma and mucoepidermoid carcinoma. Mucoepidermoid carcinoma composed of columnar cells, mucocytes, and squamoid cells showing solid and cystic lesion. Several small cysts lined by benign ciliated columnar epithelia suggesting that this tumor had originated from solid cell nest were seen around the tumor. By immunohistochemistry, columnar cells and squamoid cells in mucoepidermoid carcinoma were positive for cytokeratin but negative for thyroglobulin, TTF-1 and calcitonin. Positivity of p63 was seen in squamoid cells and basal cells of cysts. Some mucocytes are CEA positive. Tumor cells of papillary carcinoma are positive for TTF-1, thyroglobulin but negative for CEA, calcitonin and p63.
Keywords: Thyroid Gland; Carcinoma, Mucoepidermoid; Carcinoma, Papillary; Solid Cell Nests
INTRODUCTION
Mucoepidermoid carcinoma (MEC) most commonly occurs in salivary glands but can also be seen at other organs, including lung, esophagus, breast, pancreas, and thyroid gland (1-10). Primary MEC of the thyroid is very rare and its origin has not been fully understood with debate regarding whether it arises from solid cell nests of ultimobranchial apparatus or from follicular epithelium (5, 7, 10-13). Here, we present a case of unusual tumor of thyroid MEC more suggesting solid cell nest origin than follicular epithelium.
CASE REPORT
A 50-yr-old man presented with a 3-month history of a mass on the left side of his neck, which had recently increased in size on March 26, 2009.
Physical examination showed a firm, fixed mass measuring 3.0×2.5 cm. Ultrasonography and neck CT scan revealed a mass with irregular border in left lobe of thyroid gland and multiple enlargements of cervical lymph nodes. No other abnormalities were seen. Total thyroidectomy was performed. Grossly, the left lobe was almost entirely replaced by en pale yellow to tan solid mass measuring 3.0×2.5×2.0 cm. The mass was not encapsulated and showed infiltrative margin. The right lobe and the isthmus were soft tan to red without any abnormality.
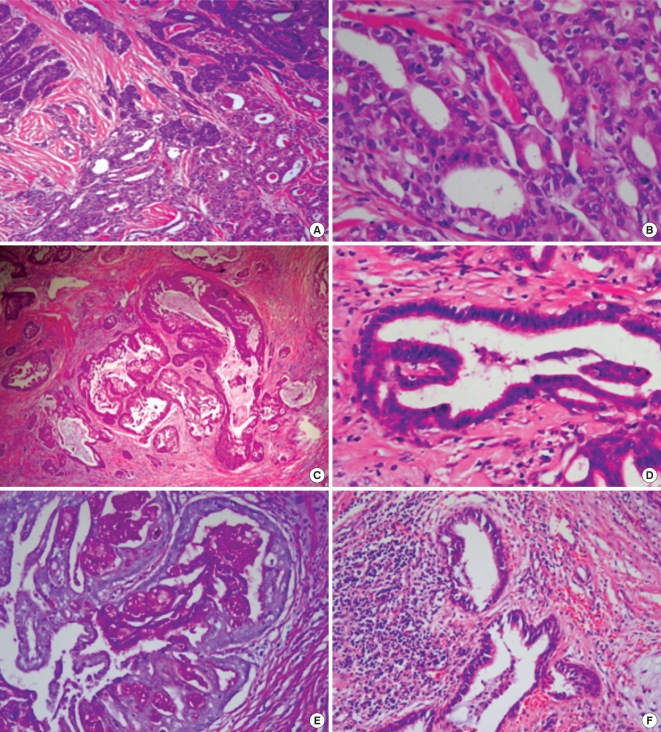
On microscopic examination, the tumor did not have fibrous capsule and infiltrated into surrounding soft tissue. The tumor was composed of follicular variant of papillary carcinoma and mucoepidermoid carcinoma. They were focally intermingled and showed transition from papillary carcinoma to squamous epithelium arranged in sheets (Fig. 1A). Papillary carcinoma tumor cells showed nuclear groove, ground glass nucleus and nuclear pseudoinclusion (Fig. 1B). Mucoepidermoid carcinoma revealed three cell types: columnar cells, mucocytes and squamous cells (Fig. 1C). Columnar cells made ductal or cystic structures and contained eosinophilic material. Some columnar cells in tumor glands showed cilia (Fig. 1D). Squamoid cells showing cytoplasmic keratinization and intercellular bridge were present in solid sheets. Alcian blue and PAS positive mucocytes were mixed with columnar and squamous cells. Eosinophilic material in ductal or cystic structures was also positive for alcian blue and PAS stains (Fig. 1E). Columnar and squamous tumor cells showed mild to moderate nuclear atypia with rare mitosis. A few aggregates of small cysts lined by benign ciliated respiratory epithelial cells and containing mucoid material are seen at the periphery of the tumor (Fig. 1F).
Fig. 1.
Histopathological findings of the tumor. (A) There are foci of transition from follicular variant of papillary carcinoma to mucoepidermoid carcinoma, H&E stained, ×40. (B) Papillary carcinoma cells show nuclear groove, ground glass nuclei and intranuclear psudoinclusions, H&E stained, ×400. (C) Mucoepidermoid carcinoma is solid and cystic lesion composed of columnar cells and squamous cells, H&E stained, ×40. (D) Columnar tumor cells have cilia, H&E stained, ×400. (E) Mucinous material in cystic space, mucocytes and cytoplasmic border of columnar cells are positive by PAS staining, ×200. (F) Small cysts lined with ciliated columnar cells (solid cell nests) are seen around the main tumor, H&E stained, ×200.
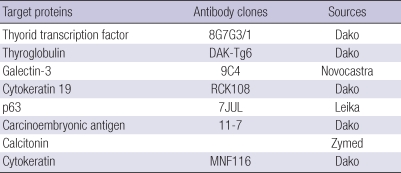
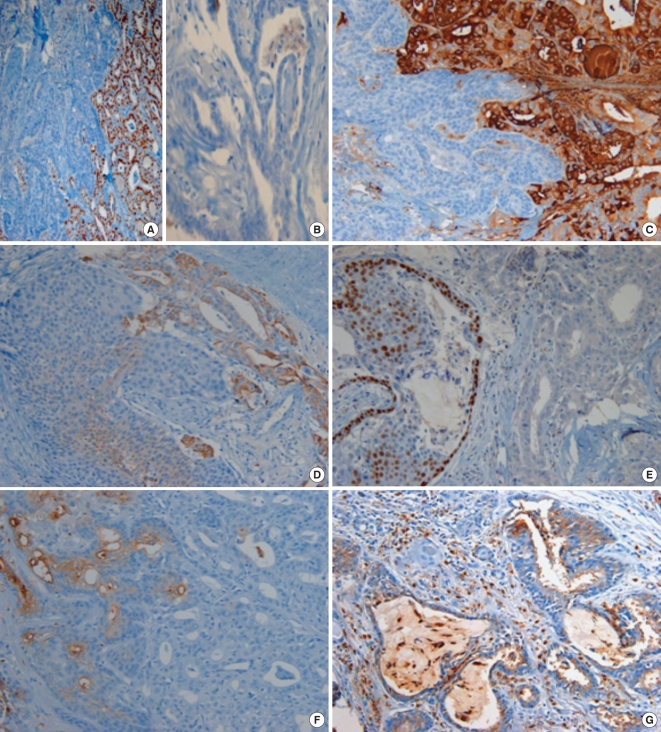
Immunohistochemical analysis was done with commercially prepared antibody panel (Table 1, Fig. 2) using EnVison Plus-HPR detection kit (Dako, Glostrup, Denmark). Papillary carcinoma cells were positive for thyroid transcription factor (TTF-1), thyroglobulin, galectin-3 and cytokeratin 19 and negative for p63, CEA and calcitonin. Squamoid and columnar cells in MEC were positive for pancytokeratin and CEA and negative for calcitonin, TTF-1, thyroglobulin, galectin-3 and cytokeratin 19. Squamoid cells and basal cells of ductal and cystic structures were positive for p63. Benign ciliated respiratory epithelial cells were CEA positive and thyroglobulin negative. The diagnosis of thyroid mucoepidermoid carcinoma combined with follicular variant of papillary carcinoma was made. Dissected neck lymph nodes revealed metastatic mucoepidermoid carcinoma. Four months after the operation, he received radiation therapy and presented dyspnea and wheezing.
Table 1.
Antibodies used in this study
Fig. 2.
Immunohistochemical findings of the tumor. Thyroid transcription factor-1 (TTF-1) was expressed in papillary carcinoma tumor cells (A) but negative in columnar cells of mucoepidermoid carcinoma (B). Papillary carcinoma tumor cells were positive for thyroglobulin (C), and cytokeratin 19 (D). Squamoid cells and basal cells of ductal structure of mucoepidermoid carcinoma were positive for p63 (E). Carcinoembryonic antigen (CEA) was expressed in squamoid and columnar tumor cells (F) and benign ciliated respiratory epithelial cells (G). (LSAB. Magnification: ×200)
DISCUSSION
MEC of the thyroid gland is a rare tumor and is characterized by unique histologic appearance and indolent biologic behavior. It was first described in 1977 by Rhatigan et al. (14) and they considered it to originate from ectopic salivary gland tissue but they couldn't find any remnants of salivary gland tissue. Its pathogenesis has remained unclear. Many authors have suggested that these tumors develop from solid cell nests (SCN), known to be remnants of ultimobranchial body (diverticulum from the fourth pharyngeal pouch), whereas some authors have reported a follicular origin (5, 7, 10-13). SCN is irregular structures of about 1 mm in maximal diameter and usually found in the thyroid lateral lobes. It is basically composed of non-keratinizing squamous cells and ductal structures lined by ciliated columnar epithelium (15). Ando et al. (10) reported MEC showing ductal structures lined by markedly ciliated columnar cells, resembling respiratory epithelial cells. The ductual structures in SCN of the ultimobranchail body also lined with ciliated epithelium and this suggests that SCN might be origin of MEC. And that is the reason tumor cells are p63 positive and TTF-1 and thyroglobulin negative. P63 is a marker of stem cell phenotype and TTF-1 and thyroglobulin are markers of thyroid follicular epithelium. These immunohistochemical results may be helpful in distinguishing ultimobranchial-derived SCN from their mimics such as squamous metaplasia of follicular cell origin tumor and metastatic squamous cell carcinoma. Although ciliated epithelium can be seen on the inner surface of thyroglossal cyst remnant but it is located on the midline. Only one another case reported by Wenig et al. (7) had focal ciliated epithelium lining cysts but that was seen in an integral parts of the neoplastic proliferation associated with squamous foci.
There are several literatures supporting a follicular epithelial origin of MEC. MEC occasionally has concurrent papillary carcinoma and histologic analysis suggests that preexisting PTC may undergo squamous and mucinous metaplasia to give rise to MEC (13, 16). MEC tumor cells are positive for thyroglobulin staining and it has been used support the follicular origin of MEC.
The present tumor is pathologically unique in that it showed ductal structures lined with ciliated columnar cells, resembling respiratory epithelium and p63 positivity suggesting SCN origin and composite papillary carcinoma component suggesting follicular origin of MEC. These findings suggest that the origin of the tumor could be stem cells of SCN and they differentiate toward MEC and papillary carcinoma. BRAF gene mutation was detected in SCN and papillary carcinoma and it was considered as the evidence of a histogenetic relation between SCN and papillary carcinoma (17). There are very limited reports about BRAF gene mutation in MEC and Trovisoco et al. (18) reported that BRAF gene mutation was not found in MEC. In this case, BRAF gene mutation was found both in PTC and MEC (not mentioned in result) but serial sections for microdissection lost SCN. More investigations of PTC, MEC and SCN at the molecular level would be helpful to find out their pathogenesis.
Clinically, thyroid MEC is most often found in female with a ratio that approximates 2:1. Although it has been described as indolent tumors with low-grade malignant potential, some reports have indicated a less favorable behavior of combined other type of carcinoma, especially with foci of undifferentiated carcinoma (19, 20). Our patient presented with combined follicular variant of papillary carcinoma and MEC and metastatic foci in cervical lymph nodes were exclusively MEC. Miranda et al. (13) reported a case of composite follicular variant of papillary carcinoma and mucoepidermoid carcinoma which showed regional lymph node metastasis. To expect the prognosis of mucoepidermoid carcinoma combined with other type of tumor, we need to find more cases.
This is the second report of MEC of thyroid gland showing ciliated glandular cells and also the second report of composite follicular variant of papillary carcinoma and mucopidermoid carcinoma of thyroid gland. These histologic and immunohistochemical findings suggest that MCE is originated from SCN rather than follicular epithelium.
References
- 1.Rossi G, Sartori G, Cavazza A, Tamberi S. Mucoepidermoid carcinoma of the lung, response to EGFR inhibitors, EGFR and K-RAS mutations, and differential diagnosis. Lung Cancer. 2009;63:159–160. doi: 10.1016/j.lungcan.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Tamura S, Kobayashi K, Seki Y, Matsuyama J, Kagara N, Ukei T, Uemura Y, Miyauchi K, Kaneko T. Mucoepidermoid carcinoma of the esophagus treated by endoscopic mucosal resection. Dis Esophagus. 2003;16:265–267. doi: 10.1046/j.1442-2050.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- 3.Markopoulos C, Gogas H, Livaditou A, Floros D. Mucoepidermoid carcinoma of the breast. Eur J Gynaecol Oncol. 1998;19:291–293. [PubMed] [Google Scholar]
- 4.Onoda N, Kang SM, Sugano S, Yamashita Y, Chung YS, Sowa M. Mucoepidermoid carcinoma of the pancreas: report of a case. Surg Today. 1995;25:843–847. doi: 10.1007/BF00311465. [DOI] [PubMed] [Google Scholar]
- 5.Arezzo A, Patetta R, Ceppa P, Borgonovo G, Torre G, Mattioli FP. Mucoepidermoid carcinoma of the thyroid gland arising from a papillary epithelial neoplasm. Am Surg. 1998;64:307–311. [PubMed] [Google Scholar]
- 6.Rodriguez-Cuevas S, Ocampo LB. A case report of mucoepidermoid carcinoma of the parotid gland developing after radioiodine therapy for thyroid carcinoma. Eur J Surg Oncol. 1995;21:692. doi: 10.1016/s0748-7983(95)96251-4. [DOI] [PubMed] [Google Scholar]
- 7.Wenig BM, Adair CF, Heffess CS. Primary mucoepidermoid carcinoma of the thyroid gland: a report of six cases and a review of the literature of a follicular epithelial-derived tumor. Hum Pathol. 1995;26:1099–1108. doi: 10.1016/0046-8177(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 8.Baloch ZW, Solomon AC, LiVolsi VA. Primary mucoepidermoid carcinoma and sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: a report of nine cases. Mod Pathol. 2000;13:802–807. doi: 10.1038/modpathol.3880140. [DOI] [PubMed] [Google Scholar]
- 9.Steele SR, Royer M, Brown TA, Porter C, Azarow KS. Mucoepidermoid carcinoma of the thyroid gland: a case report and suggested surgical approach. Am Surg. 2001;67:979–983. [PubMed] [Google Scholar]
- 10.Ando M, Nakanishi Y, Asai M, Maeshima A, Matsuno Y. Mucoepidermoid carcinoma of the thyroid gland showing marked ciliation suggestive of its pathogenesis. Pathol Int. 2008;58:741–744. doi: 10.1111/j.1440-1827.2008.02303.x. [DOI] [PubMed] [Google Scholar]
- 11.Beckner ME, Shultz JJ, Richardson T. Solid and cystic ultimobranchial body remnants in the thyroid. Arch Pathol Lab Med. 1990;114:1049–1052. [PubMed] [Google Scholar]
- 12.Reis-Filho JS, Preto A, Soares P, Ricardo S, Cameselle-Teijeiro J, Sobrinho-Simões M. p63 expression in solid cell nests of the thyroid: further evidence for a stem cell origin. Mod Pathol. 2003;16:43–48. doi: 10.1097/01.MP.0000047306.72278.39. [DOI] [PubMed] [Google Scholar]
- 13.Miranda RN, Myint MA, Gnepp DR. Composite follicular variant of papillary carcinoma and mucoepidermoid carcinoma of the thyroid. Report of a case and review of the literature. Am J Surg Pathol. 1995;19:1209–1215. doi: 10.1097/00000478-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Rhatigan RM, Roque JL, Bucher RL. Mucoepidermoid carcinoma of the thyroid gland. Cancer. 1977;39:210–214. doi: 10.1002/1097-0142(197701)39:1<210::aid-cncr2820390133>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Harach HR. Solid cell nests of the thyroid. J Pathol. 1988;155:191–200. doi: 10.1002/path.1711550303. [DOI] [PubMed] [Google Scholar]
- 16.Bhandarkar ND, Chan J, Strome M. A rare case of mucoepidermoid carcinoma of the thyroid. Am J Otolaryngol. 2005;26:138–141. doi: 10.1016/j.amjoto.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Cameselle-Teijeiro J, Abdulkader I, Perez-Becerra R, Vazquez-Boquete A, Alberte-Lista L, Ruiz-Ponte C, Forteza J, Sobrinho-Simoes M. BRAF mutation in solid cell nest hyperplasia associated with papillary thyroid carcinoma. A precursor lesion? Hum Pathol. 2009;40:1029–1035. doi: 10.1016/j.humpath.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Trovisco V, Vieira de Castro I, Soares P, Máximo V, Silva P, Magalhães J, Abrosimov A, Guiu XM, Sobrinho-Simões M. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol. 2004;202:247–251. doi: 10.1002/path.1511. [DOI] [PubMed] [Google Scholar]
- 19.Cameselle-Teijeiro J, Febles-Pérez C, Sobrinho-Simões M. Papillary and mucoepidermoid carcinoma of the thyroid with anaplastic transformation: a case report with histologic and immunohistochemical findings that support a provocative histogenetic hypothesis. Pathol Res Pract. 1995;191:1214–1221. doi: 10.1016/S0344-0338(11)81129-5. [DOI] [PubMed] [Google Scholar]
- 20.Franssila KO, Harach HR, Wasenius VM. Mucoepidermoid carcinoma of the thyroid. Histopathology. 1984;8:847–860. doi: 10.1111/j.1365-2559.1984.tb02400.x. [DOI] [PubMed] [Google Scholar]