Figure 1.
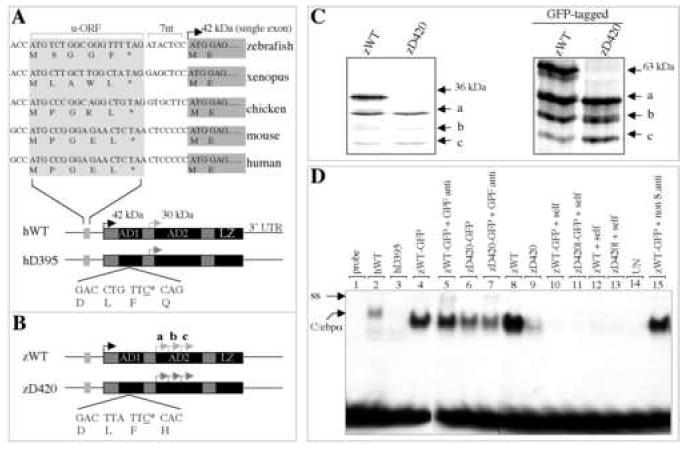
Deficiency of dominant-interfering C/ebpα isoforms in binding to human GCSFR promoter DNA. (A) Structural conservation of CEBPA gene during evolution. The deleted cytosine at position 395 of human CEBPA gene (referred to as hD395) is underlined (bottom). u-ORF, upstream of open reading frame; nt, nucleotides; AD1, transactivation domain 1; AD2, transactivation domain 2; LZ, lucine zipper; UTR, untranslated region. Black arrow denotes the translational initiation site that encodes the full-length 42-kDa C/EBPα (referred to as hWT), while gray arrow denotes the in-frame, internal initiation site that encodes a dominant-interfering, 30-kDa N-terminal truncated isoform. (B) Schematic diagram of full-length (zWT) and alternative (zD420) isoforms of zebrafish cebpa gene. The deleted cytosine homologue at position 420 of zebrafish cebpa (zD420) is underlined. Black arrow denotes the translational initiation site that encodes the full-length C/ebpα protein (36-kDa, zWT). Gray arrows mark three putative, in-frame internal initiation sites (a, b and c). (C) Proteins translated in vitro in the presence of 35S-labeled methionine from zWT and mutant zD420 (left panel), as well as GFP-tagged zWT and zD420 (right panel) expression plasmids. (D) Comparison of the ability of in vitro translated zD420 mutant proteins (lanes 6 and 9) and zWT proteins (lanes 4 and 8) to bind to the CEBP site of the human GCSFR promoter. Human wild type (hWT, lane 2) and mutant (hD395, lane 3) proteins served as positive controls. Also indicated are specific, unlabeled competitor oligonucleotides (self, lanes 10-13) used in 100-fold molar excess, unprogrammed lysates used for in vitro translation (UN, lane 14) and GFP antibody (anti, lanes 5 and 7) or nonspecific antibody (non S.anti, sheep anti-mouse IgG, lane 15) used as a negative control for supershift experiments. ss, supershift; C/ebpα, shifted band.
