Table 2.
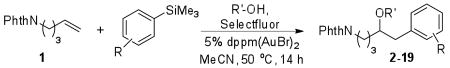 | ||||
|---|---|---|---|---|
| entry | R | R′ | product | yield (%)c |
| 1 | 4-OAc | Me | 3 | 83 |
| 2 | 4-OTf | Me | 4 | 53 |
| 3 | 4-N(Me)Ts | Me | 5 | 66 |
| 4 | 4-Me | Me | 6 | 73 |
| 5 | 4-Br | Me | 7 | 82 |
| 6 | 4-CHO | Me | 8 | 77 |
| 7 | 4-CO2Me | Me | 9 | 68 |
| 8 | 3-CO2Me | Me | 10 | 83 |
| 9 | 2-CH2CH2OH | Me | 11 | 69 |
| 10 | H | Me | 12 | 87 |
| 11 | H | Et | 2 | 83 |
| 12 | H | i-Pr | 13 | 81 |
| 13 | H | t-Bu | 14 | 37 |
| 14 | H | neopentyl | 15 | 64 |
| 15 | H | cyclopentyl | 16 | 68 |
| 16 | H | 2-methoxyethyl | 17 | 86 |
| 17 | H | H | 18 | 77 |
| 18 | 2-CH2CH2OH | H | 19 | 55 |
Conditions: 1 (100 μmol), R′OH (1.0 mmol), Selectfluor (200 μmol), ArSiMe3 (150 μmol), 5 mol % dppm(AuBr)2, 0.1 M in CH3CN, 50 °C for 14 h.
Catalyst addition in two portions over two hours.
Isolated yields.
